Assembly and Maintenance of Lipids at the Bacterial Outer Membrane
- PMID: 32955879
- PMCID: PMC7981291
- DOI: 10.1021/acs.chemrev.0c00587
Assembly and Maintenance of Lipids at the Bacterial Outer Membrane
Abstract
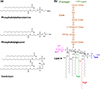
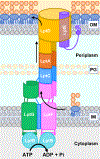
The outer membrane of Gram-negative bacteria is essential for their survival in harsh environments and provides intrinsic resistance to many antibiotics. This membrane is remarkable; it is a highly asymmetric lipid bilayer. The inner leaflet of the outer membrane contains phospholipids, whereas the fatty acyl chains attached to lipopolysaccharide (LPS) comprise the hydrophobic portion of the outer leaflet. This lipid asymmetry, and in particular the exclusion of phospholipids from the outer leaflet, is key to creating an almost impenetrable barrier to hydrophobic molecules that can otherwise pass through phospholipid bilayers. It has long been known that these lipids are not made in the outer membrane. It is now believed that conserved multisubunit protein machines extract these lipids after their synthesis is completed at the inner membrane and transport them to the outer membrane. A longstanding question is how the cell builds and maintains this asymmetric lipid bilayer in coordination with the assembly of the other components of the cell envelope. This Review describes the trans-envelope lipid transport systems that have been identified to participate in outer-membrane biogenesis: LPS transport via the Lpt machine, and phospholipid transport via the Mla pathway and several recently proposed transporters.
Figures
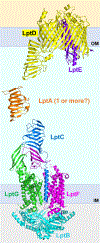



Similar articles
-
YhdP, TamB, and YdbH Are Redundant but Essential for Growth and Lipid Homeostasis of the Gram-Negative Outer Membrane.mBio. 2021 Dec 21;12(6):e0271421. doi: 10.1128/mBio.02714-21. Epub 2021 Nov 16. mBio. 2021. PMID: 34781743 Free PMC article.
-
Molecular mechanism of phospholipid transport at the bacterial outer membrane interface.Nat Commun. 2023 Dec 13;14(1):8285. doi: 10.1038/s41467-023-44144-8. Nat Commun. 2023. PMID: 38092770 Free PMC article.
-
Insight into the outer membrane asymmetry of P. aeruginosa and the role of MlaA in modulating the lipidic composition, mechanical, biophysical, and functional membrane properties of the cell envelope.Microbiol Spectr. 2024 Nov 5;12(11):e0148424. doi: 10.1128/spectrum.01484-24. Epub 2024 Oct 7. Microbiol Spectr. 2024. PMID: 39373473 Free PMC article.
-
How Bacteria Establish and Maintain Outer Membrane Lipid Asymmetry.Annu Rev Microbiol. 2024 Nov;78(1):553-573. doi: 10.1146/annurev-micro-032521-014507. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 39270665 Review.
-
Current mechanistic understanding of intermembrane lipid trafficking important for maintenance of bacterial outer membrane lipid asymmetry.Curr Opin Chem Biol. 2021 Dec;65:163-171. doi: 10.1016/j.cbpa.2021.09.004. Epub 2021 Nov 6. Curr Opin Chem Biol. 2021. PMID: 34753108 Review.
Cited by
-
Cracking outer membrane biogenesis.Biochim Biophys Acta Mol Cell Res. 2023 Feb;1870(2):119405. doi: 10.1016/j.bbamcr.2022.119405. Epub 2022 Nov 29. Biochim Biophys Acta Mol Cell Res. 2023. PMID: 36455781 Free PMC article.
-
Structure and mechanism of the bacterial lipid ABC transporter, MlaFEDB.Curr Opin Struct Biol. 2022 Oct;76:102429. doi: 10.1016/j.sbi.2022.102429. Epub 2022 Aug 15. Curr Opin Struct Biol. 2022. PMID: 35981415 Free PMC article. Review.
-
Seasonal meropenem resistance in Acinetobacter baumannii and influence of temperature-driven adaptation.BMC Microbiol. 2024 Apr 27;24(1):149. doi: 10.1186/s12866-024-03271-y. BMC Microbiol. 2024. PMID: 38678219 Free PMC article.
-
Cross-talk between phospholipid synthesis and peptidoglycan expansion by a cell wall hydrolase.Proc Natl Acad Sci U S A. 2023 Jun 13;120(24):e2300784120. doi: 10.1073/pnas.2300784120. Epub 2023 Jun 5. Proc Natl Acad Sci U S A. 2023. PMID: 37276399 Free PMC article.
-
YdbH and YnbE form an intermembrane bridge to maintain lipid homeostasis in the outer membrane of Escherichia coli.Proc Natl Acad Sci U S A. 2024 May 21;121(21):e2321512121. doi: 10.1073/pnas.2321512121. Epub 2024 May 15. Proc Natl Acad Sci U S A. 2024. PMID: 38748582 Free PMC article.
References
-
- Muhlradt PF; Golecki JR Asymmetrical distribution and artifactual reorientation of lipopolysaccharide in the outer membrane bilayer of Salmonella typhimurium. Eur. J. Biochem. 1975, 51, 343–352. - PubMed
-
- Kamio Y; Nikaido H Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry 1976, 15, 2561–2570. - PubMed
-
- Lopez-Lara IM; Geiger O Bacterial lipid diversity. Biochim. Biophys. Acta. Mol. Cell. Biol. Lipids. 2017, 1862, 1287–1299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

