Clinical relevance of low-density Plasmodium falciparum parasitemia in untreated febrile children: A cohort study
- PMID: 32956354
- PMCID: PMC7505590
- DOI: 10.1371/journal.pmed.1003318
Clinical relevance of low-density Plasmodium falciparum parasitemia in untreated febrile children: A cohort study
Abstract
Background: Low-density (LD) Plasmodium infections are missed by standard malaria rapid diagnostic tests (standard mRDT) when the blood antigen concentration is below the detection threshold. The clinical impact of these LD infections is unknown. This study investigates the clinical presentation and outcome of untreated febrile children with LD infections attending primary care facilities in a moderately endemic area of Tanzania.
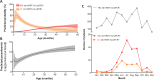
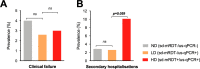
Methods/findings: This cohort study includes 2,801 febrile pediatric outpatients (median age 13.5 months [range 2-59], female:male ratio 0.8:1.0) recruited in Dar es Salaam, Tanzania between 01 December 2014 and 28 February 2016. Treatment decisions were guided by a clinical decision support algorithm run on a mobile app, which also collected clinical data. Only standard mRDT+ cases received antimalarials. Outcomes (clinical failure, secondary hospitalization, and death) were collected in follow-up visits or interviews on days 3, 7, and 28. After patient recruitment had ended, frozen blood from all 2,801 patients was tested for Plasmodium falciparum (Pf) by ultrasensitive-quantitative polymerase chain reaction (qPCR), standard mRDT, and "ultrasensitive" mRDT. As the latter did not improve sensitivity beyond standard mRDT, it is hereafter excluded. Clinical features and outcomes in LD patients (standard mRDT-/ultrasensitive-qPCR+, not given antimalarials) were compared with those with no detectable (ND) parasitemia (standard mRDT-/ultrasensitive-qPCR-) or high-density (HD) infections (standard mRDT+/ultrasensitive-qPCR+, antimalarial-treated). Pf positivity rate was 7.1% (n = 199/2,801) and 9.8% (n = 274/2,801) by standard mRDT and ultrasensitive qPCR, respectively. Thus, 28.0% (n = 76/274) of ultrasensitive qPCR+ cases were not detected by standard mRDT and labeled "LD". LD patients were, on average, 10.6 months younger than those with HD infections (95% CI 7.0-14.3 months, p < 0.001). Compared with ND, LD patients more frequently had the diagnosis of undifferentiated fever of presumed viral origin (risk ratio [RR] = 2.0, 95% CI 1.3-3.1, p = 0.003) and were more often suffering from severe malnutrition (RR = 3.2, 95% CI 1.1-7.5, p = 0.03). Despite not receiving antimalarials, outcomes for the LD group did not differ from ND regarding clinical failures (2.6% [n = 2/76] versus 4.0% [n = 101/2,527], RR = 0.7, 95% CI 0.2-3.5, p = 0.7) or secondary hospitalizations (2.6% [n = 2/76] versus 2.8% [n = 72/2,527], RR = 0.7,95% CI 0.2-3.2, p = 0.9), and no deaths were reported in any Pf-positive groups. HD patients experienced more secondary hospitalizations (10.1% [n = 20/198], RR = 0.3, 95% CI 0.1-1.0, p = 0.005) than LD patients. All the patients in this cohort were febrile children; thus, the association between parasitemia and fever cannot be investigated, nor can the conclusions be extrapolated to neonates and adults.
Conclusions: During a 28-day follow-up period, we did not find evidence of a difference in negative outcomes between febrile children with untreated LD Pf parasitemia and those without Pf parasitemia. These findings suggest LD parasitemia may either be a self-resolving fever or an incidental finding in children with other infections, including those of viral origin. These findings do not support a clinical benefit nor additional risk (e.g. because of missed bacterial infections) to using ultrasensitive malaria diagnostics at a primary care level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Ranadive N, Kunene S, Darteh S, Ntshalintshali N, Nhlabathi N, Dlamini N, et al. Limitations of Rapid Diagnostic Testing in Patients with Suspected Malaria: A Diagnostic Accuracy Evaluation from Swaziland, a Low-Endemicity Country Aiming for Malaria Elimination. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017. May 1;64(9):1221–7. 10.1093/cid/cix131 . Pubmed Central PMCID: PMC5399938. Epub 2017/04/04. - DOI - PMC - PubMed
-
- Rossi G, De Smet M, Khim N, Kindermans JM, Menard D. Performance of Rapid Diagnostic Testing in Patients with Suspected Malaria in Cambodia, a Low-Endemicity Country Aiming for Malaria Elimination. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2017. October 30;65(10):1769–70. 10.1093/cid/cix625 . Epub 2017/10/12. - DOI - PubMed
-
- Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. The Lancet infectious diseases. 2018. October;18(10):1108–16. 10.1016/S1473-3099(18)30411-0 . Epub 2018/09/02. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

