A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery
- PMID: 32956427
- PMCID: PMC7505427
- DOI: 10.1371/journal.pone.0239152
A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery
Abstract
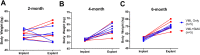
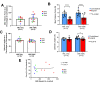
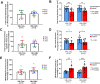
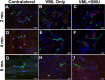
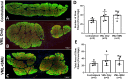
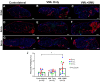
Volumetric muscle loss (VML) is the loss of skeletal muscle that results in significant and persistent impairment of function. The unique characteristics of craniofacial muscle compared trunk and limb skeletal muscle, including differences in gene expression, satellite cell phenotype, and regenerative capacity, suggest that VML injuries may affect craniofacial muscle more severely. However, despite these notable differences, there are currently no animal models of craniofacial VML. In a previous sheep hindlimb VML study, we showed that our lab's tissue engineered skeletal muscle units (SMUs) were able to restore muscle force production to a level that was statistically indistinguishable from the uninjured contralateral muscle. Thus, the goals of this study were to: 1) develop a model of craniofacial VML in a large animal model and 2) to evaluate the efficacy of our SMUs in repairing a 30% VML in the ovine zygomaticus major muscle. Overall, there was no significant difference in functional recovery between the SMU-treated group and the unrepaired control. Despite the use of the same injury and repair model used in our previous study, results showed differences in pathophysiology between craniofacial and hindlimb VML. Specifically, the craniofacial model was affected by concomitant denervation and ischemia injuries that were not exhibited in the hindlimb model. While clinically realistic, the additional ischemia and denervation likely created an injury that was too severe for our SMUs to repair. This study highlights the importance of balancing the use of a clinically realistic model while also maintaining control over variables related to the severity of the injury. These variables include the volume of muscle removed, the location of the VML injury, and the geometry of the injury, as these affect both the muscle's ability to self-regenerate as well as the probability of success of the treatment.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







Similar articles
-
Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 3-Month Recovery.Tissue Eng Part A. 2020 Aug;26(15-16):837-851. doi: 10.1089/ten.TEA.2019.0288. Epub 2020 Feb 28. Tissue Eng Part A. 2020. PMID: 32013753 Free PMC article.
-
Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 6-Month Recovery.Tissue Eng Part A. 2022 Jul;28(13-14):606-620. doi: 10.1089/ten.TEA.2021.0187. Tissue Eng Part A. 2022. PMID: 34937425 Free PMC article.
-
Repairing Volumetric Muscle Loss with Commercially Available Hydrogels in an Ovine Model.Tissue Eng Part A. 2024 May;30(9-10):440-453. doi: 10.1089/ten.TEA.2023.0240. Epub 2024 Jan 31. Tissue Eng Part A. 2024. PMID: 38117140
-
The Potential of Combination Therapeutics for More Complete Repair of Volumetric Muscle Loss Injuries: The Role of Exogenous Growth Factors and/or Progenitor Cells in Implantable Skeletal Muscle Tissue Engineering Technologies.Cells Tissues Organs. 2016;202(3-4):202-213. doi: 10.1159/000447323. Epub 2016 Nov 9. Cells Tissues Organs. 2016. PMID: 27825153 Review.
-
Vascularized and Innervated Skeletal Muscle Tissue Engineering.Adv Healthc Mater. 2020 Jan;9(1):e1900626. doi: 10.1002/adhm.201900626. Epub 2019 Oct 17. Adv Healthc Mater. 2020. PMID: 31622051 Free PMC article. Review.
Cited by
-
Impact of Cell Seeding Density and Cell Confluence on Human Tissue Engineered Skeletal Muscle.Tissue Eng Part A. 2022 May;28(9-10):420-432. doi: 10.1089/ten.TEA.2021.0132. Epub 2022 Feb 23. Tissue Eng Part A. 2022. PMID: 34652973 Free PMC article.
-
Cell-scale porosity minimizes foreign body reaction and promotes innervated myofiber formation after volumetric muscle loss.NPJ Regen Med. 2025 Mar 1;10(1):12. doi: 10.1038/s41536-025-00395-1. NPJ Regen Med. 2025. PMID: 40025057 Free PMC article.
-
Secondary denervation is a chronic pathophysiologic sequela of volumetric muscle loss.J Appl Physiol (1985). 2021 May 1;130(5):1614-1625. doi: 10.1152/japplphysiol.00049.2021. Epub 2021 Apr 8. J Appl Physiol (1985). 2021. PMID: 33830817 Free PMC article.
-
Immunomodulation and Biomaterials: Key Players to Repair Volumetric Muscle Loss.Cells. 2021 Aug 7;10(8):2016. doi: 10.3390/cells10082016. Cells. 2021. PMID: 34440785 Free PMC article. Review.
-
Extrusion-Based Printing of Myoblast-Loaded Fibrin Microthreads to Induce Myogenesis.J Funct Biomater. 2025 Jan 10;16(1):21. doi: 10.3390/jfb16010021. J Funct Biomater. 2025. PMID: 39852577 Free PMC article.
References
-
- Surgeons ASoP. 2018 National Plastic Surgery Statistics. 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

