Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma
- PMID: 32956453
- PMCID: PMC7509877
- DOI: 10.1182/bloodadvances.2020002393
Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma
Abstract
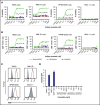
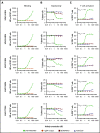
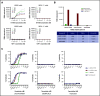
B-cell maturation antigen (BCMA), a member of the tumor necrosis factor family of receptors, is predominantly expressed on the surface of terminally differentiated B cells. BCMA is highly expressed on plasmablasts and plasma cells from multiple myeloma (MM) patient samples. We developed a BCMAxCD3 bispecific antibody (teclistamab [JNJ-64007957]) to recruit and activate T cells to kill BCMA-expressing MM cells. Teclistamab induced cytotoxicity of BCMA+ MM cell lines in vitro (H929 cells, 50% effective concentration [EC50] = 0.15 nM; MM.1R cells, EC50 = 0.06 nM; RPMI 8226 cells, EC50 = 0.45 nM) with concomitant T-cell activation (H929 cells, EC50 = 0.21 nM; MM.1R cells, EC50 = 0.1 nM; RPMI 8226 cells, EC50 = 0.28 nM) and cytokine release. This activity was further increased in the presence of a γ-secretase inhibitor (LY-411575). Teclistamab also depleted BCMA+ cells in bone marrow samples from MM patients in an ex vivo assay with an average EC50 value of 1.7 nM. Under more physiological conditions using healthy human whole blood, teclistamab mediated dose-dependent lysis of H929 cells and activation of T cells. Antitumor activity of teclistamab was also observed in 2 BCMA+ MM murine xenograft models inoculated with human T cells (tumor inhibition with H929 model and tumor regression with the RPMI 8226 model) compared with vehicle and antibody controls. The specific and potent activity of teclistamab against BCMA-expressing cells from MM cell lines, patient samples, and MM xenograft models warrant further evaluation of this bispecific antibody for the treatment of MM. Phase 1 clinical trials (monotherapy, #NCT03145181; combination therapy, #NCT04108195) are ongoing for patients with relapsed/refractory MM.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: K. Pillarisetti, A.B., X.Z., L.L., R.N., G.P., K. Packman, Y.E., R.A., and F.G. are employees of Janssen Research & Development, LLC; E.B., Y.L., M.M., N.M., and D.C. are former employees of Janssen Research & Development, LLC; and all are shareholders of Johnson & Johnson, the parent company of the Janssen Research & Development, LLC. E.B. is an employee of IBM Watson Health. Y.L. is an employee of Legend Biotech. M.M. and D.C. are employees of Century Therapeutics.
Figures




References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. - PubMed
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. . International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. - PubMed
-
- Harousseau JL, Attal M, Avet-Loiseau H, et al. . Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621-4629. - PubMed
-
- Kumar SK, Lee JH, Lahuerta JJ, et al. ; International Myeloma Working Group . Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study [published correction appears in Leukemia. 2012;26(5):1153]. Leukemia. 2012;26(1):149-157. - PMC - PubMed
-
- Lokhorst HM, Plesner T, Laubach JP, et al. . Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373(13):1207-1219. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

