The Weight of Evidence From Electrophysiology, Observational, and Cardiovascular End Point Studies Demonstrates the Safety of Azithromycin
- PMID: 32956575
- PMCID: PMC7537091
- DOI: 10.1111/cts.12867
The Weight of Evidence From Electrophysiology, Observational, and Cardiovascular End Point Studies Demonstrates the Safety of Azithromycin
Abstract
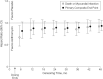
Increased use of azithromycin (AZ) in treating infections associated with coronavirus disease 2019 (COVID-19) and reports of increased incidence of prolonged corrected QT (QTc) interval associated with AZ used with hydroxychloroquine prompted us to review the latest evidence in the literature, present additional analyses of human cardiovascular (CV) electrophysiology studies, and to describe sequential steps in research and development that were undertaken to characterize the benefit-risk profile of AZ. Combined QTc findings from electrocardiograms taken during oral and i.v. pharmacokinetic-pharmacodynamic studies of AZ suggest that clinically meaningful QTc prolongation is unlikely. Findings from several observational studies were heterogeneous and not as consistent as results from at least two large randomized controlled trials (RCTs). The QTc findings presented and observational data from studies with large numbers of events are not consistent with either a proarrhythmic action of AZ or an increase in frequency of CV deaths. Well-powered RCTs do not suggest a presence of increased risk of CV or sudden cardiac death after short-term or protracted periods of AZ usage, even in patients at higher risk from pre-existing coronary disease.
© 2020 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors are employees of Pfizer, Inc.
Figures

Similar articles
-
Effects of cardiac toxicity of combination therapy with hydroxychloroquine and azithromycin in COVID-19 patients.J Infect Public Health. 2021 Nov;14(11):1668-1670. doi: 10.1016/j.jiph.2021.09.013. Epub 2021 Sep 23. J Infect Public Health. 2021. PMID: 34627063 Free PMC article.
-
QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis.Pharmacoepidemiol Drug Saf. 2021 Jun;30(6):694-706. doi: 10.1002/pds.5234. Epub 2021 Apr 3. Pharmacoepidemiol Drug Saf. 2021. PMID: 33772933 Free PMC article.
-
Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients With COVID-19.JACC Clin Electrophysiol. 2021 Jan;7(1):16-25. doi: 10.1016/j.jacep.2020.07.016. Epub 2020 Aug 5. JACC Clin Electrophysiol. 2021. PMID: 33478708 Free PMC article.
-
Investigation of QT Prolongation with Hydroxychloroquine and Azithromycin for the Treatment of COVID-19.J Coll Physicians Surg Pak. 2020 Oct;30(10):153-157. doi: 10.29271/jcpsp.2020.supp2.S153. J Coll Physicians Surg Pak. 2020. PMID: 33291194
-
Clinical Pharmacology Perspectives on the Antiviral Activity of Azithromycin and Use in COVID-19.Clin Pharmacol Ther. 2020 Aug;108(2):201-211. doi: 10.1002/cpt.1857. Epub 2020 May 12. Clin Pharmacol Ther. 2020. PMID: 32302411 Free PMC article. Review.
Cited by
-
Safety profiles and adverse reactions of azithromycin in the treatment of pediatric respiratory diseases: A systematic review and meta-analysis.Medicine (Baltimore). 2023 Dec 1;102(48):e36306. doi: 10.1097/MD.0000000000036306. Medicine (Baltimore). 2023. PMID: 38050289 Free PMC article.
-
Transmembrane protease serine 2 (TMPRSS2) rs75603675, comorbidity, and sex are the primary predictors of COVID-19 severity.Life Sci Alliance. 2022 May 30;5(10):e202201396. doi: 10.26508/lsa.202201396. Print 2022 Oct. Life Sci Alliance. 2022. PMID: 35636966 Free PMC article.
References
-
- Mecaskey, J.W. , Knirsch, C.A. , Kumaresan, J.A. & Cook, J.A. The possibility of eliminating blinding trachoma. Lancet Infect. Dis. 3, 728–734 (2003). - PubMed
-
- Knirsch, C.A. & Chandra, R. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 367, 772–773 (2012). - PubMed
-
- Crumb, W.J. Jr , Vicente, J. , Johannesen, L. & Strauss, D.G. An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J. Pharmacol. Toxicol. Methods 81, 251–262 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

