Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches
- PMID: 32956643
- PMCID: PMC7501082
- DOI: 10.1016/j.bcp.2020.114225
Role of proteolytic enzymes in the COVID-19 infection and promising therapeutic approaches
Abstract
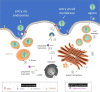
In the Fall of 2019 a sudden and dramatic outbreak of a pulmonary disease (Coronavirus Disease COVID-19), due to a new Coronavirus strain (i.e., SARS-CoV-2), emerged in the continental Chinese area of Wuhan and quickly diffused throughout the world, causing up to now several hundreds of thousand deaths. As for common viral infections, the crucial event for the viral life cycle is the entry of genetic material inside the host cell, realized by the spike protein of the virus through its binding to host receptors and its activation by host proteases; this is followed by translation of the viral RNA into a polyprotein, exploiting the host cell machinery. The production of individual mature viral proteins is pivotal for replication and release of new virions. Several proteolytic enzymes either of the host and of the virus act in a concerted fashion to regulate and coordinate specific steps of the viral replication and assembly, such as (i) the entry of the virus, (ii) the maturation of the polyprotein and (iii) the assembly of the secreted virions for further diffusion. Therefore, proteases involved in these three steps are important targets, envisaging that molecules which interfere with their activity are promising therapeutic compounds. In this review, we will survey what is known up to now on the role of specific proteolytic enzymes in these three steps and of most promising compounds designed to impair this vicious cycle.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures






References
-
- Bredenbeek P.J., Pachuk C.J., Noten A.F., Charite J., Luytjes W., Weiss S.R., Spaan W.J. The primary structure and expression of the second open reading frame of the polymerase gene of the coronavirus MHV-A59: a highly conserved polymerase is expressed by an efficient ribosomal frameshifting mechanism. Nucl. Acids Res. 1990;18:1825–1832. doi: 10.1093/nar/18.7.1825. - DOI - PMC - PubMed
-
- Lee H.J., Shieh C.K., Gorbalenya A.E., Koonin E.V., La Monica N., Tuler J., Bagdzhadzhyan A., Lai M.M. The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. Virology. 1991;180:567–582. doi: 10.1016/0042-6822(91)90071-i. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

