Growth from Birth Through Six Months for Infants of Mothers in the "Women First" Preconception Maternal Nutrition Trial
- PMID: 32956698
- PMCID: PMC7855785
- DOI: 10.1016/j.jpeds.2020.09.032
Growth from Birth Through Six Months for Infants of Mothers in the "Women First" Preconception Maternal Nutrition Trial
Abstract
Objective: To evaluate whether the fetal linear growth effects of maternal nutrition supplementation would be maintained through 6 months postnatal age.
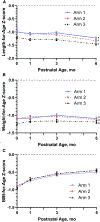
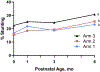
Study design: The Women First trial was a multicountry, individually randomized clinical trial that compared the impact of maternal nutrition supplementation initiated preconception (Arm 1) vs at ∼11 weeks of gestation (Arm 2), vs no supplement (Arm 3); the intervention was discontinued at delivery. Trial sites were in Democratic Republic of Congo, Guatemala, India, and Pakistan. Analysis includes 2421 infants born to 2408 randomized women. Primary outcome was the trajectory of length-for-age z scores (LAZ) by arm, based on assessments at birth and 1, 3, and 6 months. We fitted longitudinal models on growth from birth to 6 months using generalized estimating equations; maternal intervention effects were evaluated, adjusting for site and baseline maternal covariates.
Results: Linear growth for Arms 1 and 2 was statistically greater than for Arm 3 in 3 of the 4 countries, with average pairwise mean differences in LAZ of 0.25 (95% CI 0.15-0.35; P < .001) and 0.19 (95% CI 0.09-0.28; P < .001), respectively. Compared with Arm 3, average overall adjusted relative risks (95% CI) for stunting (LAZ <-2) were lower for Arms 1 and 2: 0.76 (0.66-0.87; P < .001) and 0.77 (0.67-0.88; P < .001), respectively.
Conclusions: Improved linear growth in early infancy observed for the 2 intervention arms supports the critical importance of maternal nutrition before conception and in the early phase of gestation.
Trial registration: ClinicalTrials.gov: NCT01883193.
Keywords: breastfeeding; infant growth; postnatal growth; small quantity-lipid nutrient supplement (SQ-LNS); stunting.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Comment in
-
Infant Growth After Maternal Dietary Supplementation Before and During Pregnancy.J Pediatr. 2021 Feb;229:14-16. doi: 10.1016/j.jpeds.2020.10.076. Epub 2020 Nov 4. J Pediatr. 2021. PMID: 33159913 No abstract available.
References
-
- World Bank. World Bank Annual Report 2016. Washington, DC: World Bank; 2016.
-
- World Health Organization. Global nutrition targets 2025: policy brief series (WHO/NMH/NH/14.2). Geneva: World Health Organization; 2014.
-
- United Nations Children’s Fund, World Health Organization, World Bank. Joint Child Malnutrition Estimates. Global Database on Child Growth and Malnutrition; 2015. ed2019.
-
- Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 2010;125:e473–80. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

