Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial
- PMID: 32956756
- PMCID: PMC9579892
- DOI: 10.1016/j.jaci.2020.08.037
Efficacy and safety of mepolizumab in hypereosinophilic syndrome: A phase III, randomized, placebo-controlled trial
Abstract
Background: Anti-IL-5 therapy is a potential treatment for patients with hypereosinophilic syndrome (HES), although its clinical efficacy is unclear.
Objective: We sought to investigate the clinical efficacy and safety of mepolizumab versus placebo in patients with HES.
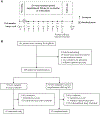
Methods: This randomized, multicenter, double-blind, placebo-controlled, phase III trial was conducted across 39 centers in 13 countries. Eligible patients had FIP1L1-PDGFRA-negative HES, experienced 2 or more flares (worsening of HES-related symptoms or blood eosinophil count requiring therapeutic escalation) in the previous 12 months, and had a screening blood eosinophil count greater than or equal to 1000 cells/μL. Patients were randomized (1:1) to subcutaneous mepolizumab (300 mg) or placebo every 4 weeks for 32 weeks, plus existing HES therapy. The primary outcome was the proportion of patients with 1 or more flares (worsening of HES-related symptoms necessitating therapy escalation or ≥2 courses of blinded rescue oral corticosteroids) during the study; in addition, patients who withdrew early from the study were counted as having a flare. Safety end points were also assessed.
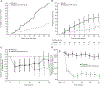
Results: The proportion of patients experiencing 1 or more flares/withdrawing from the study was 50% lower with mepolizumab versus placebo (15 of 54 [28%] vs 30 of 54 [56%]; P = .002). Logistic regression analysis was consistent with the primary analysis (odds ratio, 0.28; 95% CI, 0.12-0.64; P = .003). Similar proportions of patients in the mepolizumab and placebo groups experienced on-treatment adverse events (48 of 54 [89%] vs 47 of 54 [87%]).
Conclusions: Compared with placebo, mepolizumab significantly reduced the occurrence of flares in patients with HES, with no new safety signals identified.
Trial registration: ClinicalTrials.gov NCT02836496.
Keywords: Hypereosinophilic syndrome; efficacy; flare; mepolizumab; safety.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
IL-5-Antikörper reduziert HES-Schübe.MMW Fortschr Med. 2023 Feb;165(Suppl 1):49. doi: 10.1007/s15006-023-2382-5. MMW Fortschr Med. 2023. PMID: 36849780 German. No abstract available.
References
-
- Curtis C, Ogbogu P. Hypereosinophilic syndrome. Clin Rev Allergy Immunol 2016;50:240–51. - PubMed
-
- Shomali W, Gotlib J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am J Hematol 2019;94:1149–67. - PubMed
-
- Whitehouse MW. Anti-inflammatory glucocorticoid drugs: reflections after 60 years. Inflammopharmacology 2011;19:1–19. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

