Inositol 1,4,5-trisphosphate receptor type 3 plays a protective role in hepatocytes during hepatic ischemia-reperfusion injury
- PMID: 32957029
- PMCID: PMC7530136
- DOI: 10.1016/j.ceca.2020.102264
Inositol 1,4,5-trisphosphate receptor type 3 plays a protective role in hepatocytes during hepatic ischemia-reperfusion injury
Abstract
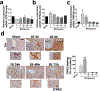
Hepatic ischemia-reperfusion injury is seen in a variety of clinical conditions, including hepatic thrombosis, systemic hypotension, and liver transplantation. Calcium (Ca2+) signaling mediates several pathophysiological processes in the liver, but it is not known whether and how intracellular Ca2+ channels are involved in the hepatocellular events secondary to ischemia-reperfusion. Using an animal model of hepatic ischemia-reperfusion injury, we observed a progressive increase in expression of the type 3 isoform of the inositol trisphosphate receptor (ITPR3), an intracellular Ca2+ channel that is not normally expressed in healthy hepatocytes. ITPR3 expression was upregulated, at least in part, by a combination of demethylation of the ITPR3 promoter region and the increased transcriptional activity of the nuclear factor of activated T-cells (NFAT). Additionally, expression of pro-inflammatory interleukins and necrotic surface area were less pronounced in livers of control animals compared to liver-specific ITPR3 KO mice subjected to hepatic damage. Corroborating these findings, ITPR3 expression and activation of NFAT were observed in hepatocytes of liver biopsies from patients who underwent liver ischemia caused by thrombosis after organ transplant. Together, these results are consistent with the idea that ITPR3 expression in hepatocytes plays a protective role during hepatic injury induced by ischemia-reperfusion.
Keywords: Calcium signaling; Hepatocytes; Necrosis; Nuclear factor of activated T-cells; Transplantation.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare that they have no known competing interest.
Figures


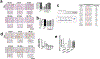



Similar articles
-
Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma.Gut. 2019 Sep;68(9):1676-1687. doi: 10.1136/gutjnl-2018-317811. Epub 2019 Jul 17. Gut. 2019. PMID: 31315892 Free PMC article.
-
Osteopontin deficiency aggravates hepatic injury induced by ischemia-reperfusion in mice.Cell Death Dis. 2014 May 8;5(5):e1208. doi: 10.1038/cddis.2014.174. Cell Death Dis. 2014. PMID: 24810044 Free PMC article.
-
Connexin 32 deficiency protects the liver against ischemia/reperfusion injury.Eur J Pharmacol. 2020 Jun 5;876:173056. doi: 10.1016/j.ejphar.2020.173056. Epub 2020 Mar 5. Eur J Pharmacol. 2020. PMID: 32147436 Clinical Trial.
-
Inositol 1,4,5-trisphosphate receptor in the liver: Expression and function.World J Gastroenterol. 2019 Nov 28;25(44):6483-6494. doi: 10.3748/wjg.v25.i44.6483. World J Gastroenterol. 2019. PMID: 31802829 Free PMC article. Review.
-
Type 3 inositol 1,4,5-trisphosphate receptor: A calcium channel for all seasons.Cell Calcium. 2020 Jan;85:102132. doi: 10.1016/j.ceca.2019.102132. Epub 2019 Nov 25. Cell Calcium. 2020. PMID: 31790953 Free PMC article. Review.
Cited by
-
Adaptation to Volumetric Compression Drives an Apoptosis-Resistant and Invasive Phenotype in Liver Cancer.Cancer Res. 2025 Aug 15;85(16):3156-3175. doi: 10.1158/0008-5472.CAN-24-0859. Cancer Res. 2025. PMID: 40387600
-
Inositol 1,4,5-trisphosphate receptor type 3 is involved in resistance to apoptosis and maintenance of human hepatocellular carcinoma.Oncol Lett. 2022 Jan;23(1):32. doi: 10.3892/ol.2021.13150. Epub 2021 Nov 25. Oncol Lett. 2022. PMID: 34966448 Free PMC article.
-
Correlation Between Clinical and Pathological Findings of Liver Injury in 27 Patients With Lethal COVID-19 Infections in Brazil.Hepatol Commun. 2022 Feb;6(2):270-280. doi: 10.1002/hep4.1820. Epub 2021 Sep 14. Hepatol Commun. 2022. PMID: 34520633 Free PMC article.
References
-
- Cannistra M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, et al. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg 2016;33 Suppl 1:S57–70. - PubMed
-
- Jaeschke H Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol 2011;26 Suppl 1:173–179. - PubMed
-
- Dhar DK, Takemoto Y, Nagasue N, Uchida M, Ono T, Nakamura T. FK506 maintains cellular calcium homeostasis in ischemia-reperfusion injury of the canine liver. J Surg Res 1996;60:142–146. - PubMed
-
- Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev 2008;88:421–449. - PubMed
-
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 1999;284:339–343. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

