Evaluation of the performance of SARS-CoV-2 serological tools and their positioning in COVID-19 diagnostic strategies
- PMID: 32957073
- PMCID: PMC7441068
- DOI: 10.1016/j.diagmicrobio.2020.115181
Evaluation of the performance of SARS-CoV-2 serological tools and their positioning in COVID-19 diagnostic strategies
Abstract
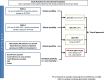
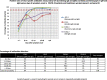
Rapid and accurate diagnosis is crucial for successful outbreak containment. During the current coronavirus disease 2019 (COVID-19) public health emergency, the gold standard for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection diagnosis is the detection of viral RNA. Additional diagnostic methods õenabling the detection of current or past SARS-CoV-2 infection would be highly beneficial. We assessed 2 immunochromatographic lateral flow assays (LFA-1, LFA-2) and 2 enzyme-linked immunosorbent assay kits (IgA/IgG ELISA-1, IgM/IgG ELISA-2) using 325 samples: serum samples from polymerase chain reaction-confirmed COVID-19 hospitalized patients (n = 55) and healthcare workers (n = 143) and 127 samples from negative controls. Diagnostic performances were assessed according to days after symptom onset (dso) and the antigenic format used by manufacturers. Clinical sensitivities varied greatly among the assays, showing poor mutual agreement. After 15 dso, ELISA-1 (Euroimmun) and LFA-1 (Biosynex) combining IgM and IgG detection showed the best performances. A thorough selection of serological assays for the detection of ongoing or past infections is advisable.
Keywords: COVID-19; Humoral response; SARS-CoV-2; Serological diagnosis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


References
-
- Burbelo P.D., Riedo F.X., Morishima C., Rawlings S., Smith D., Das S. Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J Infect Dis. 2020;222(2):206–213. doi: 10.1093/infdis/jiaa273. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

