Mucolipidoses Overview: Past, Present, and Future
- PMID: 32957425
- PMCID: PMC7555117
- DOI: 10.3390/ijms21186812
Mucolipidoses Overview: Past, Present, and Future
Abstract
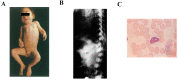
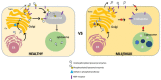
Mucolipidosis II and III (ML II/III) are caused by a deficiency of uridine-diphosphate N-acetylglucosamine: lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase (GlcNAc-1-phosphotransferase, EC2.7.8.17), which tags lysosomal enzymes with a mannose 6-phosphate (M6P) marker for transport to the lysosome. The process is performed by a sequential two-step process: first, GlcNAc-1-phosphotransferase catalyzes the transfer of GlcNAc-1-phosphate to the selected mannose residues on lysosomal enzymes in the cis-Golgi network. The second step removes GlcNAc from lysosomal enzymes by N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase (uncovering enzyme) and exposes the mannose 6-phosphate (M6P) residues in the trans-Golgi network, in which the enzymes are targeted to the lysosomes by M6Preceptors. A deficiency of GlcNAc-1-phosphotransferase causes the hypersecretion of lysosomal enzymes out of cells, resulting in a shortage of multiple lysosomal enzymes within lysosomes. Due to a lack of GlcNAc-1-phosphotransferase, the accumulation of cholesterol, phospholipids, glycosaminoglycans (GAGs), and other undegraded substrates occurs in the lysosomes. Clinically, ML II and ML III exhibit quite similar manifestations to mucopolysaccharidoses (MPSs), including specific skeletal deformities known as dysostosis multiplex and gingival hyperplasia. The life expectancy is less than 10 years in the severe type, and there is no definitive treatment for this disease. In this review, we have described the updated diagnosis and therapy on ML II/III.
Keywords: I-cell disease; glycosaminoglycans; inclusion body; lysosomal storage disorders; lysosome enzyme transport; mannose 6-phosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- National Institute of Neurological Disorders and Stroke Mucolipidoses Fact Sheet. September 2015, NIH Publication No. 15-4899. [(accessed on 29 July 2020)]; Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-She....
-
- Spranger J.W., Wiedemann H.R. The genetic mucolipidoses. Diagnosis and differential diagnosis. Humangenetik. 1970;9:113–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

