Regulation of KSHV Latency and Lytic Reactivation
- PMID: 32957532
- PMCID: PMC7551196
- DOI: 10.3390/v12091034
Regulation of KSHV Latency and Lytic Reactivation
Abstract
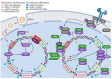
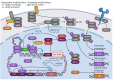
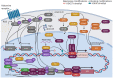
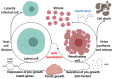
Kaposi's sarcoma-associated herpesvirus (KSHV) is associated with three malignancies- Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (MCD). Central to the pathogenesis of these diseases is the KSHV viral life cycle, which is composed of a quiescent latent phase and a replicative lytic phase. While the establishment of latency enables persistent KSHV infection and evasion of the host immune system, lytic replication is essential for the dissemination of the virus between hosts and within the host itself. The transition between these phases, known as lytic reactivation, is controlled by a complex set of environmental, host, and viral factors. The effects of these various factors converge on the regulation of two KSHV proteins whose functions facilitate each phase of the viral life cycle-latency-associated nuclear antigen (LANA) and the master switch of KSHV reactivation, replication and transcription activator (RTA). This review presents the current understanding of how the transition between the phases of the KSHV life cycle is regulated, how the various phases contribute to KSHV pathogenesis, and how the viral life cycle can be exploited as a therapeutic target.
Keywords: Kaposi’s sarcoma-associated herpesvirus (KSHV); latency; lytic cycle; reactivation; viral life cycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

