The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis
- PMID: 32957712
- PMCID: PMC7555043
- DOI: 10.3390/ijms21186837
The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis
Abstract
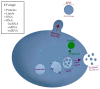
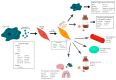
Extracellular vesicles (EVs) play a key role in the communication between cancer cells and stromal components of the tumor microenvironment (TME). In this context, cancer cell-derived EVs can regulate the activation of a CAF phenotype in TME cells, which can be mediated by several EV cargos (e.g., miRNA, proteins, mRNA and lncRNAs). On the other hand, CAF-derived EVs can mediate several processes during tumorigenesis, including tumor growth, invasion, metastasis, and therapy resistance. This review aimed to discuss the molecular aspects of EV-based cross-talk between CAFs and cancer cells during tumorigenesis, in addition to assessing the roles of EV cargo in therapy resistance and pre-metastatic niche formation.
Keywords: cancer-associated fibroblasts; extracellular vesicles; neoplasms; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts.Mol Cancer. 2019 Dec 3;18(1):175. doi: 10.1186/s12943-019-1101-4. Mol Cancer. 2019. PMID: 31796058 Free PMC article.
-
Cancer associated-fibroblast-derived exosomes in cancer progression.Mol Cancer. 2021 Dec 1;20(1):154. doi: 10.1186/s12943-021-01463-y. Mol Cancer. 2021. PMID: 34852849 Free PMC article. Review.
-
Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis.Cells. 2021 Dec 6;10(12):3429. doi: 10.3390/cells10123429. Cells. 2021. PMID: 34943937 Free PMC article. Review.
-
Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance.OMICS. 2020 Jun;24(6):314-339. doi: 10.1089/omi.2020.0023. OMICS. 2020. PMID: 32496970
-
Extracellular vesicles released by hypoxia-induced tumor-associated fibroblasts impart chemoresistance to breast cancer cells via long noncoding RNA H19 delivery.FASEB J. 2024 Jan 31;38(2):e23165. doi: 10.1096/fj.202300203R. FASEB J. 2024. PMID: 38197195
Cited by
-
Fibroblasts in cancer: Unity in heterogeneity.Cell. 2023 Apr 13;186(8):1580-1609. doi: 10.1016/j.cell.2023.03.016. Cell. 2023. PMID: 37059066 Free PMC article. Review.
-
MicroRNAs in Metastasis and the Tumour Microenvironment.Int J Mol Sci. 2021 May 4;22(9):4859. doi: 10.3390/ijms22094859. Int J Mol Sci. 2021. PMID: 34064331 Free PMC article. Review.
-
Cancer-associated fibroblast-derived extracellular vesicles promote lymph node metastases in oral cavity squamous cell carcinoma by encapsulating ITGB1 and BMI1.BMC Cancer. 2024 Jan 22;24(1):113. doi: 10.1186/s12885-024-11855-0. BMC Cancer. 2024. PMID: 38254031 Free PMC article.
-
Editorial: A year in review: discussions in adrenal endocrinology.Front Endocrinol (Lausanne). 2023 Sep 22;14:1291582. doi: 10.3389/fendo.2023.1291582. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37810878 Free PMC article. No abstract available.
-
Re-Shaping the Pancreatic Cancer Tumor Microenvironment: A New Role for the Metastasis Suppressor NDRG1.Cancers (Basel). 2023 May 16;15(10):2779. doi: 10.3390/cancers15102779. Cancers (Basel). 2023. PMID: 37345116 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

