Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method
- PMID: 32957733
- PMCID: PMC7560244
- DOI: 10.3390/ma13184147
Impact of Gadolinium on the Structure and Magnetic Properties of Nanocrystalline Powders of Iron Oxides Produced by the Extraction-Pyrolytic Method
Abstract
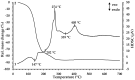
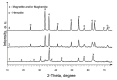
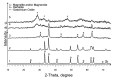
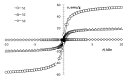
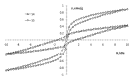
Interest in magnetic nanoparticles is primarily due to their practical use. In this work, for the production of nanocrystalline powders of pure and gadolinium doped iron oxides, the extraction-pyrolytic method (EPM) was used. As a precursor, either iron-containing extract (iron (III) caproate in caproic acid) or its mixture with gadolinium-containing extract (gadolinium (III) valerate in valeric acid) was used. The mixed precursor contained 0.5 mol %, 2.5 mol %, 12.5 mol %, 50 mol %, and 75 mol % gadolinium in relation to the iron content. The formation of iron oxide phases, depending on the preparation conditions, was investigated. According to the results obtained, it was demonstrated that the presence of more than 2.5 mol % gadolinium additive in the mixed precursor inhibits the magnetite-to-hematite transformation process during thermal treatment. Produced samples were characterized by XRD and SEM methods, and the magnetic properties were studied.
Keywords: coercivity; extraction–pyrolitic method; gadolinium impact; iron oxides; magnetization; nanostructures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Podkovyrina Y.S., Kremennaya M.A., Soldatov M.A., Soldatov A. Influence of Local Atomic and Electronic Structures of Magnetite on Subtle Effects in HERFD-XANES Spectra. J. Struct. Chem. 2018;59:1362–1367. doi: 10.1134/S002247661806015X. - DOI
-
- Gubin S.P., A Koksharov Y., Khomutov G., Yurkov G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005;74:489–520. doi: 10.1070/RC2005v074n06ABEH000897. - DOI
-
- Zbořil R., Mashlan M., Petridis D. Iron(III) Oxides from Thermal ProcessesSynthesis, Structural and Magnetic Properties, Mössbauer Spectroscopy Characterization, and Applications. Chem. Mater. 2002;14:969–982. doi: 10.1021/cm0111074. - DOI
-
- Sidhu P.S. Transformation of Trace Element-Substituted Maghemite to Hematite. Clays Clay Miner. 1988;36:31–38. doi: 10.1346/CCMN.1988.0360105. - DOI
-
- Haneda K., Morrish A.H. Magnetite to maghemite transformation in ultrafine particles. J. Phys. Colloq. 1977;38:321–323. doi: 10.1051/jphyscol:1977166. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

