Anti-Telomerase CD4+ Th1 Immunity and Monocytic-Myeloid-Derived-Suppressor Cells Are Associated with Long-Term Efficacy Achieved by Docetaxel, Cisplatin, and 5-Fluorouracil (DCF) in Advanced Anal Squamous Cell Carcinoma: Translational Study of Epitopes-HPV01 and 02 Trials
- PMID: 32957741
- PMCID: PMC7554943
- DOI: 10.3390/ijms21186838
Anti-Telomerase CD4+ Th1 Immunity and Monocytic-Myeloid-Derived-Suppressor Cells Are Associated with Long-Term Efficacy Achieved by Docetaxel, Cisplatin, and 5-Fluorouracil (DCF) in Advanced Anal Squamous Cell Carcinoma: Translational Study of Epitopes-HPV01 and 02 Trials
Abstract
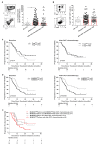
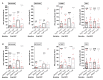
Docetaxel, cisplatin and 5-fluorouracil (DCF) chemotherapy regimen is highly effective in advanced anal squamous cell carcinoma (SCCA), as demonstrated by the Epitopes-HPV02 study results. Here, we analyzed the impact of DCF regimen and the prognostic value of adaptive immune responses and immunosuppressive cells in SCCA patients included in two prospective studies (Epitopes-HPV01 and HPV02). The presence of T-cell responses against Human papillomavirus (HPV)16-E6/E7 and anti-telomerase (hTERT)-antigens was measured by IFNᵧ-ELISpot. Here, we showed that HPV-adaptive immune responses are increased in SCCA patients. SCCA patients also displayed enhanced circulating TH1 T-cells restricted by hTERT. Exposition to DCF increased hTERT immunity but not HPV or common viruses immune responses. Notably, the correlation of hTERT immune responses with SCCA patients' clinical outcomes highlights that hTERT is a relevant antigen in this HPV-related disease. The influence of peripheral immunosuppressive cells was investigated by flow cytometry. While both regulatory T-cells and monocytic-myeloid-derived suppressive cells (M-MDSC) accumulated in the peripheral blood of SCCA patients, only high levels of M-MDSC were negatively correlated with hTERT adaptive immune responses and predicted poor prognosis. Altogether, our results reveal that hTERT is a relevant antigen in HPV-driven SCCA disease and that M-MDSC levels influence TH1-adaptive immune responses and patients' survival.
Keywords: Biomarkers; DCF chemotherapy; M-MDSC; advanced anal squamous cell carcinoma; hTERT antigens.
Conflict of interest statement
C.B. declared these conflicts of interest: Advisory board: MSD, Pierre Fabre, Roche, Research grant: Roche; D.V. declared these conflicts of interest: Advisory board: Haliodx, Cellprothera, Incyte, Merck Serono. All remaining authors have declared no conflicts of interest.
Figures


References
-
- Kim S.C., François E., André T., Samalin E., Jary M., El Hajbi F., Baba-Hamed N., Pernot S., Kaminsky M.-C., Bouché O., et al. Docetaxel, cisplatin, and fluorouracil chemotherapy for metastatic or unresectable locally recurrent anal squamous cell carcinoma (Epitopes-HPV02): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2018;19:1094–1106. doi: 10.1016/S1470-2045(18)30321-8. - DOI - PubMed
-
- Kim S., Meurisse A., Stouvenot M., Jary M., Hon T.N.T., Francois E., Buecher B., Andre T., Samalin E., Boulbair F., et al. Updated data of epitopes-HPV02 trial and external validation of efficacy of DCF in prospective epitopes-HPV01 study in advanced anal squamous cell carcinoma. Pooled analysis of 115 patients. Ann. Oncol. 2019;30:v203. doi: 10.1093/annonc/mdz246.012. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

