Identification of lipid biomarker from serum in patients with chronic obstructive pulmonary disease
- PMID: 32957957
- PMCID: PMC7507726
- DOI: 10.1186/s12931-020-01507-9
Identification of lipid biomarker from serum in patients with chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States with no effective treatment. The current diagnostic method, spirometry, does not accurately reflect the severity of COPD disease status. Therefore, there is a pressing unmet medical need to develop noninvasive methods and reliable biomarkers to detect early stages of COPD. Lipids are the fundamental components of cell membranes, and dysregulation of lipids was proven to be associated with COPD. Lipidomics is a comprehensive approach to all the pathways and networks of cellular lipids in biological systems. It is widely used for disease diagnosis, biomarker identification, and pathology disorders detection relating to lipid metabolism.
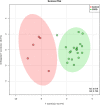
Methods: In the current study, a total of 25 serum samples were collected from 5 normal control subjects and 20 patients with different stages of COPD according to the global initiative for chronic obstructive lung disease (GOLD) (GOLD stages I ~ IV, 5 patients per group). After metabolite extraction, lipidomic analysis was performed using electrospray ionization mass spectrometry (ESI-MS) to detect the serum lipid species. Later, the comparisons of individual lipids were performed between controls and patients with COPD. Orthogonal projections to latent structures discriminant analysis (OPLS-DA) and receiver operating characteristic (ROC) analysis were utilized to test the potential biomarkers. Finally, correlations between the validated lipidomic biomarkers and disease stages, age, FEV1% pack years and BMI were evaluated.
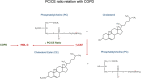
Results: Our results indicate that a panel of 50 lipid metabolites including phospholipids, sphingolipids, glycerolipids, and cholesterol esters can be used to differentiate the presence of COPD. Among them, 10 individual lipid species showed significance (p < 0.05) with a two-fold change. In addition, lipid ratios between every two lipid species were also evaluated as potential biomarkers. Further multivariate data analysis and receiver operating characteristic (ROC: 0.83 ~ 0.99) analysis suggest that four lipid species (AUC:0.86 ~ 0.95) and ten lipid ratios could be potential biomarkers for COPD (AUC:0.94 ~ 1) with higher sensitivity and specificity. Further correlation analyses indicate these potential biomarkers were not affected age, BMI, stages and FEV1%, but were associated with smoking pack years.
Conclusion: Using lipidomics and statistical methods, we identified unique lipid signatures as potential biomarkers for diagnosis of COPD. Further validation studies of these potential biomarkers with large population may elucidate their roles in the development of COPD.
Keywords: Biomarkers; Chronic obstructive pulmonary disease (COPD); Lipidomics; OPLS-DA; Receiver operating characteristic.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Plasma Metabolomics and Lipidomics Reveal Perturbed Metabolites in Different Disease Stages of Chronic Obstructive Pulmonary Disease.Int J Chron Obstruct Pulmon Dis. 2020 Mar 9;15:553-565. doi: 10.2147/COPD.S229505. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32210549 Free PMC article.
-
Identification of the lipid biomarkers from plasma in idiopathic pulmonary fibrosis by Lipidomics.BMC Pulm Med. 2017 Dec 6;17(1):174. doi: 10.1186/s12890-017-0513-4. BMC Pulm Med. 2017. PMID: 29212488 Free PMC article.
-
Study of the metabolomic relationship between lung cancer and chronic obstructive pulmonary disease based on direct infusion mass spectrometry.Biochimie. 2019 Feb;157:111-122. doi: 10.1016/j.biochi.2018.11.007. Epub 2018 Nov 13. Biochimie. 2019. PMID: 30439409
-
The clinical value of suPAR in diagnosis and prediction for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis.Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620938546. doi: 10.1177/1753466620938546. Ther Adv Respir Dis. 2020. PMID: 32643535 Free PMC article.
-
Mass spectrometric approaches in discovering lipid biomarkers for COVID-19 by lipidomics: Future challenges and perspectives.Mass Spectrom Rev. 2024 Sep-Oct;43(5):1041-1065. doi: 10.1002/mas.21848. Epub 2023 May 8. Mass Spectrom Rev. 2024. PMID: 37102760 Review.
Cited by
-
Computational Analysis of Plasma Lipidomics from Mice Fed Standard Chow and Ketogenic Diet.Bio Protoc. 2023 Sep 20;13(18):e4819. doi: 10.21769/BioProtoc.4819. eCollection 2023 Sep 20. Bio Protoc. 2023. PMID: 37753463 Free PMC article.
-
Metabolomic Analysis of Respiratory Epithelial Lining Fluid in Patients with Chronic Obstructive Pulmonary Disease-A Systematic Review.Cells. 2023 Mar 8;12(6):833. doi: 10.3390/cells12060833. Cells. 2023. PMID: 36980173 Free PMC article.
-
Metabolome Features of COPD: A Scoping Review.Metabolites. 2022 Jul 5;12(7):621. doi: 10.3390/metabo12070621. Metabolites. 2022. PMID: 35888745 Free PMC article.
-
Non-Exosomal and Exosome-Derived miRNAs as Promising Biomarkers in Canine Mammary Cancer.Life (Basel). 2022 Apr 1;12(4):524. doi: 10.3390/life12040524. Life (Basel). 2022. PMID: 35455015 Free PMC article. Review.
-
The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis.Int J Mol Sci. 2021 Jun 23;22(13):6711. doi: 10.3390/ijms22136711. Int J Mol Sci. 2021. PMID: 34201488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

