Optimising triage procedures for patients with cancer needing active anticancer treatment in the COVID-19 era
- PMID: 32958531
- PMCID: PMC7507249
- DOI: 10.1136/esmoopen-2020-000885
Optimising triage procedures for patients with cancer needing active anticancer treatment in the COVID-19 era
Abstract
Background: Immunosuppression induced by anticancer therapy in a COVID-19-positive asymptomatic patient with cancer may have a devastating effect and, eventually, be lethal. To identify asymptomatic cases among patients receiving active cancer treatment, the Federico II University Hospital in Naples performs rapid serological tests in addition to hospital standard clinical triage for COVID-19 infection.
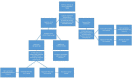
Methods: From 6 to 17 April 2020, all candidates for chemotherapy, radiotherapy or target/immunotherapy, if negative at the standard clinical triage on the day scheduled for anticancer treatment, received a rapid serological test on peripheral blood for COVID-19 IgM and IgG detection. In case of COVID-19 IgM and/or IgG positivity, patients underwent a real-time PCR (RT-PCR) SARS-CoV-2 test to confirm infection, and active cancer treatment was delayed.
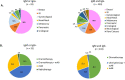
Results: Overall 466 patients, negative for COVID-19 symptoms, underwent serological testing in addition to standard clinical triage. The average age was 61 years (range 25-88 years). Most patients (190, 40.8%) had breast cancer, and chemotherapy with or without immunotherapy was administered in 323 (69.3%) patients. Overall 433 (92.9%) patients were IgG-negative and IgM-negative, and 33 (7.1%) were IgM-positive and/or IgG-positive. Among the latter patients, 18 (3.9%), 11 (2.4%) and 4 (0.9%) were IgM-negative/IgG-positive, IgM-positive/IgG-negative and IgM-positive/IgG-positive, respectively. All 33 patients with a positive serological test, tested negative for RT-PCR SARS-CoV-2 test. No patient in our cohort developed symptoms suggestive of active COVID-19 infection.
Conclusion: Rapid serological testing at hospital admission failed to detect active asymptomatic COVID-19 infection. Moreover, it entailed additional economic and human resources, delayed therapy administrationand increased hospital accesses.
Keywords: SARS-CoV-2; cancer; covid-19.
© Author (s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. Published by BMJ on behalf of the European Society for Medical Oncology.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- COVID-19 coronavirus pandemic. Available: https://www.worldometers.info/coronavirus/
-
- Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi:10.1016/S1470-2045(20)30096-6 - DOI - PMC - PubMed
-
- Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi:10.1016/S0140-6736(20)30566-3 - DOI - PMC - PubMed
-
- Onder G., Rezza G., Brusaferro S. JAMA. 2020. Case-Fatality rate and characteristics of patients dying in relation to COVID-19 in Italy.http://www.ncbi.nlm.nih.gov/pubmed/32203977doi:10.1001/jama.2020.4683 doi [Epub ahead of print 23 Mar 2020] - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

