The palmitoyl acyltransferases ZDHHC5 and ZDHHC8 are uniquely present in DRG axons and control retrograde signaling via the Gp130/JAK/STAT3 pathway
- PMID: 32958558
- PMCID: PMC7667964
- DOI: 10.1074/jbc.RA120.013815
The palmitoyl acyltransferases ZDHHC5 and ZDHHC8 are uniquely present in DRG axons and control retrograde signaling via the Gp130/JAK/STAT3 pathway
Abstract
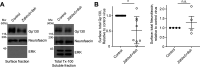
Palmitoylation, the modification of proteins with the lipid palmitate, is a key regulator of protein targeting and trafficking. However, knowledge of the roles of specific palmitoyl acyltransferases (PATs), which catalyze palmitoylation, is incomplete. For example, little is known about which PATs are present in neuronal axons, although long-distance trafficking of palmitoyl-proteins is important for axon integrity and for axon-to-soma retrograde signaling, a process critical for axon development and for responses to injury. Identifying axonally targeted PATs might thus provide insights into multiple aspects of axonal biology. We therefore comprehensively determined the subcellular distribution of mammalian PATs in dorsal root ganglion (DRG) neurons and, strikingly, found that only two PATs, ZDHHC5 and ZDHHC8, were enriched in DRG axons. Signals via the Gp130/JAK/STAT3 and DLK/JNK pathways are important for axonal injury responses, and we found that ZDHHC5 and ZDHHC8 were required for Gp130/JAK/STAT3, but not DLK/JNK, axon-to-soma signaling. ZDHHC5 and ZDHHC8 robustly palmitoylated Gp130 in cotransfected nonneuronal cells, supporting the possibility that Gp130 is a direct ZDHHC5/8 substrate. In DRG neurons, Zdhhc5/8 shRNA knockdown reduced Gp130 palmitoylation and even more markedly reduced Gp130 surface expression, potentially explaining the importance of these PATs for Gp130-dependent signaling. Together, these findings provide new insights into the subcellular distribution and roles of specific PATs and reveal a novel mechanism by which palmitoylation controls axonal retrograde signaling.
Keywords: DLK; DRG; axon; intracellular trafficking; neuron; protein acylation; protein kinase; protein palmitoylation.
© 2020 Collura et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Coupled Control of Distal Axon Integrity and Somal Responses to Axonal Damage by the Palmitoyl Acyltransferase ZDHHC17.Cell Rep. 2020 Nov 17;33(7):108365. doi: 10.1016/j.celrep.2020.108365. Cell Rep. 2020. PMID: 33207199 Free PMC article.
-
Palmitoylation-dependent control of JAK1 kinase signaling governs responses to neuropoietic cytokines and survival in DRG neurons.J Biol Chem. 2023 Aug;299(8):104965. doi: 10.1016/j.jbc.2023.104965. Epub 2023 Jun 24. J Biol Chem. 2023. PMID: 37356718 Free PMC article.
-
Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):763-8. doi: 10.1073/pnas.1514123113. Epub 2015 Dec 30. Proc Natl Acad Sci U S A. 2016. PMID: 26719418 Free PMC article.
-
Spatial organization of palmitoyl acyl transferases governs substrate localization and function.Mol Membr Biol. 2019 Dec;35(1):60-75. doi: 10.1080/09687688.2019.1710274. Mol Membr Biol. 2019. PMID: 31969037 Free PMC article. Review.
-
Putting proteins in their place: palmitoylation in Huntington disease and other neuropsychiatric diseases.Prog Neurobiol. 2012 May;97(2):220-38. doi: 10.1016/j.pneurobio.2011.11.002. Epub 2011 Dec 7. Prog Neurobiol. 2012. PMID: 22155432 Review.
Cited by
-
Regulation of pattern recognition receptor signaling by palmitoylation.iScience. 2024 Dec 20;28(2):111667. doi: 10.1016/j.isci.2024.111667. eCollection 2025 Feb 21. iScience. 2024. PMID: 39877903 Free PMC article. Review.
-
Coupled Control of Distal Axon Integrity and Somal Responses to Axonal Damage by the Palmitoyl Acyltransferase ZDHHC17.Cell Rep. 2020 Nov 17;33(7):108365. doi: 10.1016/j.celrep.2020.108365. Cell Rep. 2020. PMID: 33207199 Free PMC article.
-
S-palmitoylation: a novel insight in the development and immunotherapy of oral squamous cell carcinoma.J Cancer. 2025 Jun 12;16(9):2787-2799. doi: 10.7150/jca.110721. eCollection 2025. J Cancer. 2025. PMID: 40657375 Free PMC article. Review.
-
Fat traffic control: S-acylation in axonal transport.Mol Pharmacol. 2025 Jun;107(6):100039. doi: 10.1016/j.molpha.2025.100039. Epub 2025 Apr 16. Mol Pharmacol. 2025. PMID: 40349611 Free PMC article. Review.
-
Identification of substrates of palmitoyl protein thioesterase 1 highlights roles of depalmitoylation in disulfide bond formation and synaptic function.PLoS Biol. 2022 Mar 31;20(3):e3001590. doi: 10.1371/journal.pbio.3001590. eCollection 2022 Mar. PLoS Biol. 2022. PMID: 35358180 Free PMC article.
References
-
- Sanders S. S., Martin D. D., Butland S. L., Lavallée-Adam M., Calzolari D., Kay C., Yates J. R. 3rd,., and Hayden M. R. (2015) Curation of the mammalian palmitoylome indicates a pivotal role for palmitoylation in diseases and disorders of the nervous system and cancers. PLoS Comput. Biol. 11, e1004405 10.1371/journal.pcbi.1004405 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

