Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years
- PMID: 32958581
- PMCID: PMC7857410
- DOI: 10.1126/science.abd7343
Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years
Abstract
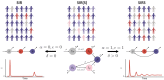
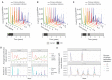
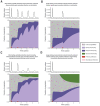
The future trajectory of the coronavirus disease 2019 (COVID-19) pandemic hinges on the dynamics of adaptive immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); however, salient features of the immune response elicited by natural infection or vaccination are still uncertain. We use simple epidemiological models to explore estimates for the magnitude and timing of future COVID-19 cases, given different assumptions regarding the protective efficacy and duration of the adaptive immune response to SARS-CoV-2, as well as its interaction with vaccines and nonpharmaceutical interventions. We find that variations in the immune response to primary SARS-CoV-2 infections and a potential vaccine can lead to markedly different immune landscapes and burdens of critically severe cases, ranging from sustained epidemics to near elimination. Our findings illustrate likely complexities in future COVID-19 dynamics and highlight the importance of immunological characterization beyond the measurement of active infections for adequately projecting the immune landscape generated by SARS-CoV-2 infections.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

