Implications for tetraspanin-enriched microdomain assembly based on structures of CD9 with EWI-F
- PMID: 32958604
- PMCID: PMC7536822
- DOI: 10.26508/lsa.202000883
Implications for tetraspanin-enriched microdomain assembly based on structures of CD9 with EWI-F
Abstract
Tetraspanins are eukaryotic membrane proteins that contribute to a variety of signaling processes by organizing partner-receptor molecules in the plasma membrane. How tetraspanins bind and cluster partner receptors into tetraspanin-enriched microdomains is unknown. Here, we present crystal structures of the large extracellular loop of CD9 bound to nanobodies 4C8 and 4E8 and, the cryo-EM structure of 4C8-bound CD9 in complex with its partner EWI-F. CD9-EWI-F displays a tetrameric arrangement with two central EWI-F molecules, dimerized through their ectodomains, and two CD9 molecules, one bound to each EWI-F transmembrane helix through CD9-helices h3 and h4. In the crystal structures, nanobodies 4C8 and 4E8 bind CD9 at loops C and D, which is in agreement with the 4C8 conformation in the CD9-EWI-F complex. The complex varies from nearly twofold symmetric (with the two CD9 copies nearly anti-parallel) to ca. 50° bent arrangements. This flexible arrangement of CD9-EWI-F with potential CD9 homo-dimerization at either end provides a "concatenation model" for forming short linear or circular assemblies, which may explain the occurrence of tetraspanin-enriched microdomains.
© 2020 Oosterheert et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


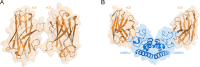







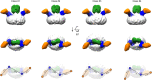
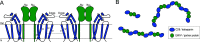
References
-
- Adams PD, Grosse-Kunstleve RW, Hung L-W, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954. 10.1107/S0907444902016657 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
