Human G-MDSCs are neutrophils at distinct maturation stages promoting tumor growth in breast cancer
- PMID: 32958605
- PMCID: PMC7536824
- DOI: 10.26508/lsa.202000893
Human G-MDSCs are neutrophils at distinct maturation stages promoting tumor growth in breast cancer
Abstract
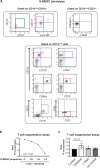
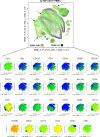
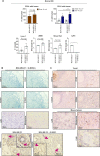
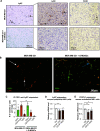
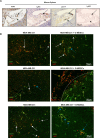
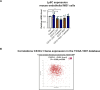
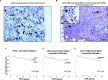
Myeloid-derived suppressor cells (MDSCs) are known to contribute to immune evasion in cancer. However, the function of the human granulocytic (G)-MDSC subset during tumor progression is largely unknown, and there are no established markers for their identification in human tumor specimens. Using gene expression profiling, mass cytometry, and tumor microarrays, we here demonstrate that human G-MDSCs occur as neutrophils at distinct maturation stages, with a disease-specific profile. G-MDSCs derived from patients with metastatic breast cancer and malignant melanoma display a unique immature neutrophil profile, that is more similar to healthy donor neutrophils than to G-MDSCs from sepsis patients. Finally, we show that primary G-MDSCs from metastatic breast cancer patients co-transplanted with breast cancer cells, promote tumor growth, and affect vessel formation, leading to myeloid immune cell exclusion. Our findings reveal a role for human G-MDSC in tumor progression and have clinical implications also for targeted immunotherapy.
© 2020 Mehmeti-Ajradini et al.
Conflict of interest statement
K Leandersson is a board member of Cantargia AB, a company developing IL1RAP inhibitors. This does not alter the author’s adherence to all guidelines for publication. The authors otherwise declare no competing interest.
Figures





References
-
- Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z, Anderson RL, Ethier SP, Jirstrom K, Pahlman S, et al. (2016) Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat Commun 7: 13050 10.1038/ncomms13050 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
