A Self-Assembling and Disassembling (SADA) Bispecific Antibody (BsAb) Platform for Curative Two-step Pretargeted Radioimmunotherapy
- PMID: 32958698
- PMCID: PMC7855367
- DOI: 10.1158/1078-0432.CCR-20-2150
A Self-Assembling and Disassembling (SADA) Bispecific Antibody (BsAb) Platform for Curative Two-step Pretargeted Radioimmunotherapy
Abstract
Purpose: Many cancer treatments suffer from dose-limiting toxicities to vital organs due to poor therapeutic indices. To overcome these challenges we developed a novel multimerization platform that rapidly removes tumor-targeting proteins from the blood to substantially improve therapeutic index.
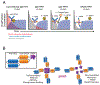
Experimental design: The platform was designed as a fusion of a self-assembling and disassembling (SADA) domain to a tandem single-chain bispecific antibody (BsAb, anti-ganglioside GD2 × anti-DOTA). SADA-BsAbs were assessed with multiple in vivo tumor models using two-step pretargeted radioimmunotherapy (PRIT) to evaluate tumor uptake, dosimetry, and antitumor responses.
Results: SADA-BsAbs self-assembled into stable tetramers (220 kDa), but could also disassemble into dimers or monomers (55 kDa) that rapidly cleared via renal filtration and substantially reduced immunogenicity in mice. When used with rapidly clearing DOTA-caged PET isotopes, SADA-BsAbs demonstrated accurate tumor localization, dosimetry, and improved imaging contrast by PET/CT. When combined with therapeutic isotopes, two-step SADA-PRIT safely delivered massive doses of alpha-emitting (225Ac, 1.48 MBq/kg) or beta-emitting (177Lu, 6,660 MBq/kg) S-2-(4-aminobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA) payloads to tumors, ablating them without any short-term or long-term toxicities to the bone marrow, kidneys, or liver.
Conclusions: The SADA-BsAb platform safely delivered large doses of radioisotopes to tumors and demonstrated no toxicities to the bone marrow, kidneys, or liver. Because of its modularity, SADA-BsAbs can be easily adapted to most tumor antigens, tumor types, or drug delivery approaches to improve therapeutic index and maximize the delivered dose.See related commentary by Capala and Kunos, p. 377.
©2020 American Association for Cancer Research.
Figures





Comment in
-
A New Generation of "Magic Bullets" for Molecular Targeting of Cancer.Clin Cancer Res. 2021 Jan 15;27(2):377-379. doi: 10.1158/1078-0432.CCR-20-3690. Epub 2020 Nov 3. Clin Cancer Res. 2021. PMID: 33144340
Comment on
-
A New Generation of "Magic Bullets" for Molecular Targeting of Cancer.Clin Cancer Res. 2021 Jan 15;27(2):377-379. doi: 10.1158/1078-0432.CCR-20-3690. Epub 2020 Nov 3. Clin Cancer Res. 2021. PMID: 33144340
References
-
- Cheal SM, Xu H, Guo HF, Lee SG, Punzalan B, Chalasani S, et al. Theranostic pretargeted radioimmunotherapy of colorectal cancer xenografts in mice using picomolar affinity (8)(6)Y- or (1)(7)(7)Lu-DOTA-Bn binding scFv C825/GPA33 IgG bispecific immunoconjugates. Eur J Nucl Med Mol Imaging 2016;43(5):925–37 doi 10.1007/s00259-015-3254-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

