A Cyclic Phosphoramidate Prodrug of 2'-Deoxy-2'-Fluoro-2'- C-Methylguanosine for the Treatment of Dengue Virus Infection
- PMID: 32958712
- PMCID: PMC7674056
- DOI: 10.1128/AAC.00654-20
A Cyclic Phosphoramidate Prodrug of 2'-Deoxy-2'-Fluoro-2'- C-Methylguanosine for the Treatment of Dengue Virus Infection
Abstract
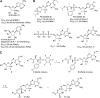
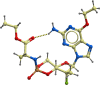
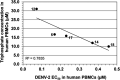
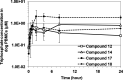
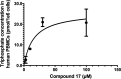
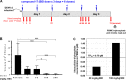
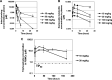
Monophosphate prodrug analogs of 2'-deoxy-2'-fluoro-2'-C-methylguanosine have been reported as potent inhibitors of hepatitis C virus (HCV) RNA-dependent RNA polymerase. These prodrugs also display potent anti-dengue virus activities in cellular assays although their prodrug moieties were designed to produce high levels of triphosphate in the liver. Since peripheral blood mononuclear cells (PBMCs) are among the major targets of dengue virus, different prodrug moieties were designed to effectively deliver 2'-deoxy-2'-fluoro-2'-C-methylguanosine monophosphate prodrugs and their corresponding triphosphates into PBMCs after oral administration. We identified a cyclic phosphoramidate, prodrug 17, demonstrating well-balanced anti-dengue virus cellular activity and in vitro stability profiles. We further determined the PBMC concentration of active triphosphate needed to inhibit virus replication by 50% (TP50). Compound 17 was assessed in an AG129 mouse model and demonstrated 1.6- and 2.2-log viremia reductions at 100 and 300 mg/kg twice a day (BID), respectively. At 100 mg/kg BID, the terminal triphosphate concentration in PBMCs exceeded the TP50 value, demonstrating TP50 as the target exposure for efficacy. In dogs, oral administration of compound 17 resulted in high PBMC triphosphate levels, exceeding the TP50 at 10 mg/kg. Unfortunately, 2-week dog toxicity studies at 30, 100, and 300 mg/kg/day showed that "no observed adverse effect level" (NOAEL) could not be achieved due to pulmonary inflammation and hemorrhage. The preclinical safety results suspended further development of compound 17. Nevertheless, present work has proven the concept that an efficacious monophosphate nucleoside prodrug could be developed for the potential treatment of dengue virus infection.
Keywords: cyclic phosphoramidate; dengue; monophosphate prodrug; nucleoside triphosphate; nucleotide analog; polymerase inhibitor.
Copyright © 2020 American Society for Microbiology.
Figures
Similar articles
-
Evaluation of AT-752, a Double Prodrug of a Guanosine Nucleotide Analog with In Vitro and In Vivo Activity against Dengue and Other Flaviviruses.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0098821. doi: 10.1128/AAC.00988-21. Epub 2021 Aug 23. Antimicrob Agents Chemother. 2021. PMID: 34424050 Free PMC article.
-
Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus.PLoS One. 2020 Jan 8;15(1):e0227104. doi: 10.1371/journal.pone.0227104. eCollection 2020. PLoS One. 2020. PMID: 31914458 Free PMC article.
-
Structure-activity relationship of uridine-based nucleoside phosphoramidate prodrugs for inhibition of dengue virus RNA-dependent RNA polymerase.Bioorg Med Chem Lett. 2018 Jul 15;28(13):2324-2327. doi: 10.1016/j.bmcl.2018.04.069. Epub 2018 May 3. Bioorg Med Chem Lett. 2018. PMID: 29801997
-
The search for nucleoside/nucleotide analog inhibitors of dengue virus.Antiviral Res. 2015 Oct;122:12-9. doi: 10.1016/j.antiviral.2015.07.010. Epub 2015 Aug 1. Antiviral Res. 2015. PMID: 26241002 Review.
-
2'-Fluoro-6'-methylene carbocyclic adenosine and its phosphoramidate prodrug: A novel anti-HBV agent, active against drug-resistant HBV mutants.Med Res Rev. 2018 May;38(3):977-1002. doi: 10.1002/med.21490. Epub 2018 Feb 6. Med Res Rev. 2018. PMID: 29406612 Review.
Cited by
-
Finding a chink in the armor: Update, limitations, and challenges toward successful antivirals against flaviviruses.PLoS Negl Trop Dis. 2022 Apr 28;16(4):e0010291. doi: 10.1371/journal.pntd.0010291. eCollection 2022 Apr. PLoS Negl Trop Dis. 2022. PMID: 35482672 Free PMC article. Review.
-
A Comprehensive Review of the Development and Therapeutic Use of Antivirals in Flavivirus Infection.Viruses. 2025 Jan 8;17(1):74. doi: 10.3390/v17010074. Viruses. 2025. PMID: 39861863 Free PMC article. Review.
-
Novel Prodrug Strategies for the Treatment of Tuberculosis.Chem Asian J. 2024 Dec 2;19(23):e202400944. doi: 10.1002/asia.202400944. Epub 2024 Oct 24. Chem Asian J. 2024. PMID: 39179514 Free PMC article. Review.
-
Prodrugs of Nucleoside 5'-Monophosphate Analogues: Overview of the Recent Literature Concerning their Synthesis and Applications.Curr Med Chem. 2023;30(11):1256-1303. doi: 10.2174/0929867329666220909122820. Curr Med Chem. 2023. PMID: 36093825 Review.
-
Evaluation of AT-752, a Double Prodrug of a Guanosine Nucleotide Analog with In Vitro and In Vivo Activity against Dengue and Other Flaviviruses.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0098821. doi: 10.1128/AAC.00988-21. Epub 2021 Aug 23. Antimicrob Agents Chemother. 2021. PMID: 34424050 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi:10.1038/nature12060. - DOI - PMC - PubMed
-
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon IK, van der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A, CYD14 Study Group. 2014. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384:1358–1365. doi:10.1016/S0140-6736(14)61060-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

