GPR101 drives growth hormone hypersecretion and gigantism in mice via constitutive activation of Gs and Gq/11
- PMID: 32958754
- PMCID: PMC7506554
- DOI: 10.1038/s41467-020-18500-x
GPR101 drives growth hormone hypersecretion and gigantism in mice via constitutive activation of Gs and Gq/11
Abstract
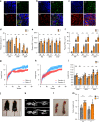
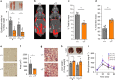
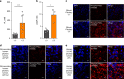
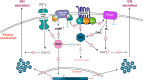
Growth hormone (GH) is a key modulator of growth and GH over-secretion can lead to gigantism. One form is X-linked acrogigantism (X-LAG), in which infants develop GH-secreting pituitary tumors over-expressing the orphan G-protein coupled receptor, GPR101. The role of GPR101 in GH secretion remains obscure. We studied GPR101 signaling pathways and their effects in HEK293 and rat pituitary GH3 cell lines, human tumors and in transgenic mice with elevated somatotrope Gpr101 expression driven by the rat Ghrhr promoter (GhrhrGpr101). Here, we report that Gpr101 causes elevated GH/prolactin secretion in transgenic GhrhrGpr101 mice but without hyperplasia/tumorigenesis. We show that GPR101 constitutively activates not only Gs, but also Gq/11 and G12/13, which leads to GH secretion but not proliferation. These signatures of GPR101 signaling, notably PKC activation, are also present in human pituitary tumors with high GPR101 expression. These results underline a role for GPR101 in the regulation of somatotrope axis function.
Conflict of interest statement
The authors declare the following competing interests A.F.D. and A.B. are Inventors of granted US Patent No. 10,350, 273B2, Treatment of Hormonal Disorders of Growth. All other authors declare no competing interests.
Figures




References
-
- Buchman M, Bell S, Kopchick JJ. Growth hormone discovery and structure. Pediatr. Endocrinol. Rev. 2018;16:2–10. - PubMed
-
- Steyn, F. J., Tolle, V., Chen, C. & Epelbaum, J. In Comprehensive Physiology vol. 6, pp. 687–735 (John Wiley & Sons, Inc., 2016). - PubMed
-
- Beckers A, Petrossians P, Hanson J, Daly AF. The causes and consequences of pituitary gigantism. Nat. Rev. Endocrinol. 2018;14:705–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

