Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer
- PMID: 32958762
- PMCID: PMC7506026
- DOI: 10.1038/s41467-020-18557-8
Ring-opening functionalizations of unstrained cyclic amines enabled by difluorocarbene transfer
Abstract
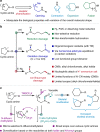
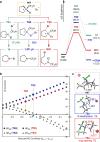
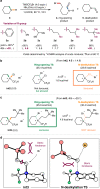
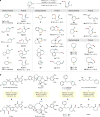
Chemical synthesis based on the skeletal variation has been prolifically utilized as an attractive approach for modification of molecular properties. Given the ubiquity of unstrained cyclic amines, the ability to directly alter such motifs would grant an efficient platform to access unique chemical space. Here, we report a highly efficient and practical strategy that enables the selective ring-opening functionalization of unstrained cyclic amines. The use of difluorocarbene leads to a wide variety of multifaceted acyclic architectures, which can be further diversified to a range of distinctive homologative cyclic scaffolds. The virtue of this deconstructive strategy is demonstrated by successful modification of several natural products and pharmaceutical analogues.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Roughley SD, Jordan AM. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011;54:3451–3479. - PubMed
-
- Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J. Med. Chem. 2014;57:5845–5859. - PubMed
-
- Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. - PubMed
-
- Blakemore DC, et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018;10:383–394. - PubMed
-
- Beak P, Kerrick ST, Wu S, Chu J. Complex induced proximity effects: enantioselective syntheses based on asymmetric deprotonations of N-Boc-pyrrolidines. J. Am. Chem. Soc. 1994;116:3231–3239.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

