CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis
- PMID: 32958780
- PMCID: PMC7505845
- DOI: 10.1038/s41467-020-18565-8
CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis
Abstract
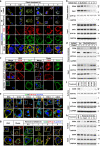
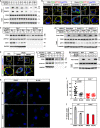
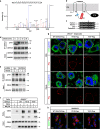
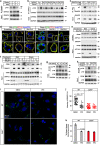
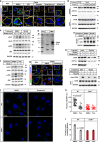
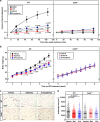
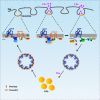
Fatty acids (FAs) are essential nutrients, but how they are transported into cells remains unclear. Here, we show that FAs trigger caveolae-dependent CD36 internalization, which in turn delivers FAs into adipocytes. During the process, binding of FAs to CD36 activates its downstream kinase LYN, which phosphorylates DHHC5, the palmitoyl acyltransferase of CD36, at Tyr91 and inactivates it. CD36 then gets depalmitoylated by APT1 and recruits another tyrosine kinase SYK to phosphorylate JNK and VAVs to initiate endocytic uptake of FAs. Blocking CD36 internalization by inhibiting APT1, LYN or SYK abolishes CD36-dependent FA uptake. Restricting CD36 at either palmitoylated or depalmitoylated state eliminates its FA uptake activity, indicating an essential role of dynamic palmitoylation of CD36. Furthermore, blocking endocytosis by targeting LYN or SYK inhibits CD36-dependent lipid droplet growth in adipocytes and high-fat-diet induced weight gain in mice. Our study has uncovered a dynamic palmitoylation-regulated endocytic pathway to take up FAs.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
DHHC4 and DHHC5 Facilitate Fatty Acid Uptake by Palmitoylating and Targeting CD36 to the Plasma Membrane.Cell Rep. 2019 Jan 2;26(1):209-221.e5. doi: 10.1016/j.celrep.2018.12.022. Cell Rep. 2019. PMID: 30605677
-
FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts.Mol Biol Cell. 2005 Jan;16(1):24-31. doi: 10.1091/mbc.e04-07-0616. Epub 2004 Oct 20. Mol Biol Cell. 2005. PMID: 15496455 Free PMC article.
-
Conjugated linoleic acid (CLA) reduces intestinal fatty acid uptake and chylomicron formation in HFD-fed mice associated with the inhibition of DHHC7-mediated CD36 palmitoylation and the downstream ERK pathway.Food Funct. 2024 May 7;15(9):5000-5011. doi: 10.1039/d4fo00099d. Food Funct. 2024. PMID: 38618651
-
Cellular fatty acid uptake: a pathway under construction.Trends Endocrinol Metab. 2009 Mar;20(2):72-7. doi: 10.1016/j.tem.2008.11.001. Epub 2009 Jan 29. Trends Endocrinol Metab. 2009. PMID: 19185504 Free PMC article. Review.
-
Context-specific fatty acid uptake is a finely-tuned multi-level effort.Trends Endocrinol Metab. 2025 Jun;36(6):577-590. doi: 10.1016/j.tem.2024.10.001. Epub 2024 Oct 25. Trends Endocrinol Metab. 2025. PMID: 39490380 Review.
Cited by
-
CD36 Senses Dietary Lipids and Regulates Lipids Homeostasis in the Intestine.Front Physiol. 2021 Apr 28;12:669279. doi: 10.3389/fphys.2021.669279. eCollection 2021. Front Physiol. 2021. PMID: 33995128 Free PMC article. Review.
-
Targeting CD36-Mediated Lipid Metabolism by Selective Inhibitor-Augmented Antitumor Immune Responses in Oral Cancer.Int J Mol Sci. 2024 Aug 30;25(17):9438. doi: 10.3390/ijms25179438. Int J Mol Sci. 2024. PMID: 39273384 Free PMC article.
-
Shigella flexneri remodeling and consumption of host lipids during infection.J Bacteriol. 2023 Dec 19;205(12):e0032023. doi: 10.1128/jb.00320-23. Epub 2023 Nov 22. J Bacteriol. 2023. PMID: 37991380 Free PMC article.
-
Fatty Acid Metabolism in Endothelial Cell.Genes (Basel). 2022 Dec 6;13(12):2301. doi: 10.3390/genes13122301. Genes (Basel). 2022. PMID: 36553568 Free PMC article. Review.
-
Scd1 Deficiency in Early Embryos Affects Blastocyst ICM Formation through RPs-Mdm2-p53 Pathway.Int J Mol Sci. 2023 Jan 16;24(2):1750. doi: 10.3390/ijms24021750. Int J Mol Sci. 2023. PMID: 36675264 Free PMC article.
References
-
- McArthur MJ, et al. Cellular uptake and intracellular trafficking of long chain fatty acids. J. Lipid Res. 1999;40:1371–1383. - PubMed
-
- Hamilton JA. Fatty acid transport: difficult or easy? J. Lipid Res. 1998;39:467–481. - PubMed
-
- Hamilton JA. Fast flip-flop of cholesterol and fatty acids in membranes: implications for membrane transport proteins. Curr. Opin. Lipidol. 2003;14:263–271. - PubMed
-
- Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu. Rev. Nutr. 2002;22:383–415. - PubMed
-
- Ehehalt R, et al. Translocation of long chain fatty acids across the plasma membrane–lipid rafts and fatty acid transport proteins. Mol. Cell. Biochem. 2006;284:135–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

