Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19
- PMID: 32959052
- PMCID: PMC7702482
- DOI: 10.1182/blood.2020008423
Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19
Abstract
Anti-CD20 monoclonal antibodies are widely used for the treatment of hematological malignancies or autoimmune disease but may be responsible for a secondary humoral deficiency. In the context of COVID-19 infection, this may prevent the elicitation of a specific SARS-CoV-2 antibody response. We report a series of 17 consecutive patients with profound B-cell lymphopenia and prolonged COVID-19 symptoms, negative immunoglobulin G (IgG)-IgM SARS-CoV-2 serology, and positive RNAemia measured by digital polymerase chain reaction who were treated with 4 units of COVID-19 convalescent plasma. Within 48 hours of transfusion, all but 1 patient experienced an improvement of clinical symptoms. The inflammatory syndrome abated within a week. Only 1 patient who needed mechanical ventilation for severe COVID-19 disease died of bacterial pneumonia. SARS-CoV-2 RNAemia decreased to below the sensitivity threshold in all 9 evaluated patients. In 3 patients, virus-specific T-cell responses were analyzed using T-cell enzyme-linked immunospot assay before convalescent plasma transfusion. All showed a maintained SARS-CoV-2 T-cell response and poor cross-response to other coronaviruses. No adverse event was reported. Convalescent plasma with anti-SARS-CoV-2 antibodies appears to be a very promising approach in the context of protracted COVID-19 symptoms in patients unable to mount a specific humoral response to SARS-CoV-2.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
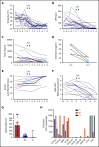
Comment in
-
COVID-19, plasma, and hypogammaglobulinemia.Blood. 2020 Nov 12;136(20):2245-2246. doi: 10.1182/blood.2020008963. Blood. 2020. PMID: 33180920 Free PMC article.
References
-
- Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS trial group . Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471. - PubMed
-
- Hauser SL, Waubant E, Arnold DL, et al. ; HERMES Trial Group . B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. - PubMed
-
- Sacco KA, Abraham RS. Consequences of B-cell-depleting therapy: hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy. 2018;10(8):713-728. - PubMed
-
- Avouac J, Airó P, Carlier N, Matucci-Cerinic M, Allanore Y. Severe COVID-19-associated pneumonia in 3 patients with systemic sclerosis treated with rituximab [published online ahead of print 5 June 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217864217864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

