Synthesis, Structure-Activity Relationship, and Antimalarial Efficacy of 6-Chloro-2-arylvinylquinolines
- PMID: 32959656
- PMCID: PMC7605213
- DOI: 10.1021/acs.jmedchem.0c00858
Synthesis, Structure-Activity Relationship, and Antimalarial Efficacy of 6-Chloro-2-arylvinylquinolines
Abstract
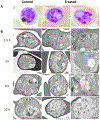
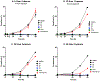
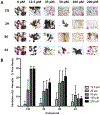
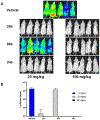
There is an urgent need to develop new efficacious antimalarials to address the emerging drug-resistant clinical cases. Our previous phenotypic screening identified styrylquinoline UCF501 as a promising antimalarial compound. To optimize UCF501, we herein report a detailed structure-activity relationship study of 2-arylvinylquinolines, leading to the discovery of potent, low nanomolar antiplasmodial compounds against a Plasmodium falciparum CQ-resistant Dd2 strain, with excellent selectivity profiles (resistance index < 1 and selectivity index > 200). Several metabolically stable 2-arylvinylquinolines are identified as fast-acting agents that kill asexual blood-stage parasites at the trophozoite phase, and the most promising compound 24 also demonstrates transmission blocking potential. Additionally, the monophosphate salt of 24 exhibits excellent in vivo antimalarial efficacy in the murine model without noticeable toxicity. Thus, the 2-arylvinylquinolines represent a promising class of antimalarial drug leads.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
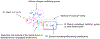

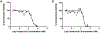


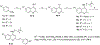
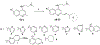

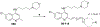

References
-
- WHO. World Malaria Report, 2019.
-
- Mishra M; Mishra VK; Kashaw V; Iyer AK; Kashaw SK Comprehensive review on various strategies for antimalarial drug discovery. Eur. J. Med. Chem. 2017, 125, 1300–1320. - PubMed
-
- Tilley L; Rosenthal PJ Malaria parasites fine-tune mutations to resist drugs. Nature 2019, 576, 217–219. - PubMed
-
- Stocks PA; Raynes KJ; Bray PG; Park BK; O’Neill PM; Ward SA Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J. Med. Chem. 2002, 45, 4975–4983. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

