Nicotine stimulates ion transport via metabotropic β4 subunit containing nicotinic ACh receptors
- PMID: 32959891
- PMCID: PMC7707097
- DOI: 10.1111/bph.15270
Nicotine stimulates ion transport via metabotropic β4 subunit containing nicotinic ACh receptors
Abstract
Background and purpose: Mucociliary clearance is an innate immune process of the airways, essential for removal of respiratory pathogens. It depends on ciliary beat and ion and fluid homeostasis of the epithelium. We have shown that nicotinic ACh receptors (nAChRs) activate ion transport in mouse tracheal epithelium. Yet the receptor subtypes and signalling pathways involved remained unknown.
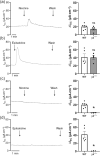
Experimental approach: Transepithelial short circuit currents (ISC ) of freshly isolated mouse tracheae were recorded using the Ussing chamber technique. Changes in [Ca2+ ]i were studied on freshly dissociated mouse tracheal epithelial cells.
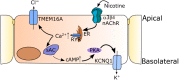
Key results: Apical application of the nAChR agonist nicotine transiently increased ISC . The nicotine effect was abolished by the nAChR antagonist mecamylamine. α-Bungarotoxin (α7 antagonist) had no effect. The agonists epibatidine (α3β2, α4β2, α4β4 and α3β4) and A-85380 (α4β2 and α3β4) increased ISC . The antagonists dihydro-β-erythroidine (α4β2, α3β2, α4β4 and α3β4), α-conotoxin MII (α3β2) and α-conotoxin PnIA (α3β2) reduced the nicotine effect. Nicotine- and epibatidine-induced currents were unaltered in β2-/- mice, but in β4-/- mice no increase was observed. In the presence of thapsigargin (endoplasmatic reticulum Ca2+ -ATPase inhibitor) or the ryanodine receptor antagonists JTV-519 and dantrolene there was a reduction in the nicotine-effect, indicating involvement of Ca2+ release from intracellular stores. Additionally, the PKA inhibitor H-89 and the TMEM16A (Ca2+ -activated chloride channel) inhibitor T16Ainh-A01 significantly reduced the nicotine-effect.
Conclusion and implications: α3β4 nAChRs are responsible for the nicotine-induced current changes via Ca2+ release from intracellular stores, PKA and ryanodine receptor activation. These nAChRs might be possible targets to stimulate chloride transport via TMEM16A.
Keywords: ACh receptors; Ussing chamber; epithelium; nicotine; non-neuronal cholinergic system; trachea.
© 2020 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Chavez‐Noriega, L. E. , Crona, J. H. , Washburn, M. S. , Urrutia, A. , Elliott, K. J. , & Johnson, E. C. (1997). Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. The Journal of Pharmacology and Experimental Therapeutics, 280, 346–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

