Explorations into the Effect of meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials
- PMID: 32959963
- PMCID: PMC7985877
- DOI: 10.1002/anie.202008075
Explorations into the Effect of meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials
Abstract
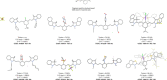
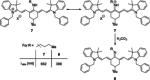
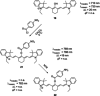
Polymethine cyanine dyes have been widely recognized as promising chemical tools for a range of life science and biomedical applications, such as fluorescent staining of DNA and proteins in gel electrophoresis, fluorescence guided surgery, or as ratiometric probes for probing biochemical pathways. The photophysical properties of such dyes can be tuned through the synthetic modification of the conjugated backbone, for example, by altering aromatic cores or by varying the length of the conjugated polymethine chain. Alternative routes to shaping the absorption, emission, and photostability of dyes of this family are centered around the chemical modifications on the polymethine chain. This Minireview aims to discuss strategies for the introduction of substituents in the meso-position, their effect on the photophysical properties of these dyes and some structure-activity correlations which could help overcome common limitations in the state of the art in the synthesis.
Keywords: chemical probes; cyanine; fluorescence; imaging agent; meso-substitution.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Symmetric Meso-Chloro-Substituted Pentamethine Cyanine Dyes Containing Benzothiazolyl/Benzoselenazolyl Chromophores Novel Synthetic Approach and Studies on Photophysical Properties upon Interaction with bio-Objects.J Fluoresc. 2016 Jan;26(1):177-87. doi: 10.1007/s10895-015-1700-4. Epub 2015 Nov 2. J Fluoresc. 2016. PMID: 26521968
-
Synthesis and evaluation of cyanine-styryl dyes with enhanced photostability for fluorescent DNA staining.Org Biomol Chem. 2013 Nov 21;11(43):7458-62. doi: 10.1039/c3ob41717d. Org Biomol Chem. 2013. PMID: 24088963
-
Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments.Q Rev Biophys. 2011 Feb;44(1):123-51. doi: 10.1017/S0033583510000247. Epub 2010 Nov 26. Q Rev Biophys. 2011. PMID: 21108866 Review.
-
Synthesis and Optical Properties of Near-Infrared meso-Phenyl-Substituted Symmetric Heptamethine Cyanine Dyes.Molecules. 2018 Jan 24;23(2):226. doi: 10.3390/molecules23020226. Molecules. 2018. PMID: 29364846 Free PMC article.
-
Productive Manipulation of Cyanine Dye π-Networks.Angew Chem Int Ed Engl. 2019 Jul 1;58(27):8974-8976. doi: 10.1002/anie.201902956. Epub 2019 May 24. Angew Chem Int Ed Engl. 2019. PMID: 31124257 Free PMC article. Review.
Cited by
-
Doubly Strapped Zwitterionic NIR-I and NIR-II Heptamethine Cyanine Dyes for Bioconjugation and Fluorescence Imaging.Angew Chem Int Ed Engl. 2023 Jul 10;62(28):e202305062. doi: 10.1002/anie.202305062. Epub 2023 May 31. Angew Chem Int Ed Engl. 2023. PMID: 37163228 Free PMC article.
-
Unraveling the Chemistry of meso-Cl Tricarbocyanine Dyes in Conjugation Reactions for the Creation of Peptide Bonds.ACS Bio Med Chem Au. 2022 Dec 21;2(6):642-654. doi: 10.1021/acsbiomedchemau.2c00053. Epub 2022 Nov 8. ACS Bio Med Chem Au. 2022. PMID: 36573095 Free PMC article.
-
Photoactive monofunctional Pt(ii)-cyanine complex for nucleus and mitochondria dual-targeted antitumor therapy.Chem Sci. 2025 Jun 26;16(33):15066-15074. doi: 10.1039/d5sc01616a. eCollection 2025 Aug 20. Chem Sci. 2025. PMID: 40698155 Free PMC article.
-
Functional Diversity in Radiolabeled Nanoceramics and Related Biomaterials for the Multimodal Imaging of Tumors.ACS Bio Med Chem Au. 2023 Aug 8;3(5):389-417. doi: 10.1021/acsbiomedchemau.3c00021. eCollection 2023 Oct 18. ACS Bio Med Chem Au. 2023. PMID: 37876497 Free PMC article. Review.
-
Fluorescent Water-Soluble Polycationic Chitosan Polymers as Markers for Biological 3D Imaging.Chem Biomed Imaging. 2024 Sep 10;2(10):721-730. doi: 10.1021/cbmi.4c00028. eCollection 2024 Oct 28. Chem Biomed Imaging. 2024. PMID: 39483637 Free PMC article.
References
-
- None
-
- Gray R., Walton D., Bickerton J., Richards P., Heptinstall J., Dyes Pigm. 1998, 38, 97–105;
-
- Pauli J., Vag T., Haag R., Spieles M., Wenzel M., Kaiser W. A., Resch-Genger U., Hilger I., Eur. J. Med. Chem. 2009, 44, 3496–3503; - PubMed
-
- Mujumdar S. R., Mujumdar R. B., Grant C. M., Waggoner A. S., Bioconjugate Chem. 1996, 7, 356–362; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

