Explorations into the Effect of meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials
- PMID: 32959963
- PMCID: PMC7985877
- DOI: 10.1002/anie.202008075
Explorations into the Effect of meso-Substituents in Tricarbocyanine Dyes: A Path to Diverse Biomolecular Probes and Materials
Abstract
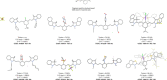
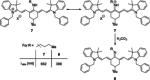
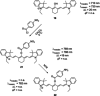
Polymethine cyanine dyes have been widely recognized as promising chemical tools for a range of life science and biomedical applications, such as fluorescent staining of DNA and proteins in gel electrophoresis, fluorescence guided surgery, or as ratiometric probes for probing biochemical pathways. The photophysical properties of such dyes can be tuned through the synthetic modification of the conjugated backbone, for example, by altering aromatic cores or by varying the length of the conjugated polymethine chain. Alternative routes to shaping the absorption, emission, and photostability of dyes of this family are centered around the chemical modifications on the polymethine chain. This Minireview aims to discuss strategies for the introduction of substituents in the meso-position, their effect on the photophysical properties of these dyes and some structure-activity correlations which could help overcome common limitations in the state of the art in the synthesis.
Keywords: chemical probes; cyanine; fluorescence; imaging agent; meso-substitution.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- None
-
- Gray R., Walton D., Bickerton J., Richards P., Heptinstall J., Dyes Pigm. 1998, 38, 97–105;
-
- Pauli J., Vag T., Haag R., Spieles M., Wenzel M., Kaiser W. A., Resch-Genger U., Hilger I., Eur. J. Med. Chem. 2009, 44, 3496–3503; - PubMed
-
- Mujumdar S. R., Mujumdar R. B., Grant C. M., Waggoner A. S., Bioconjugate Chem. 1996, 7, 356–362; - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

