CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos
- PMID: 32959976
- PMCID: PMC7576282
- DOI: 10.1111/acel.13240
CXCL5-CXCR2 signaling is a senescence-associated secretory phenotype in preimplantation embryos
Abstract
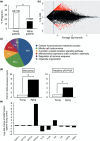
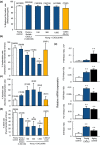
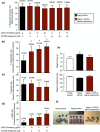
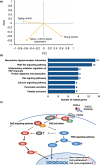
Pregnancy rate of women decreases with age due to declining quality of oocytes and embryos. However, there is no established method to improve pregnancy rate in aging women. In this study, we identified a senescence-associated secretory phenotype (SASP) factor partially responsible for the decline in embryo implantation potential. Based on microarray analysis using young and aging human embryos at the same morphological grade, 702 genes showed >fivefold increases in aging human blastocysts. Among these genes, C-X-C motif chemokine 5 (CXCL5) showed 7.7-fold increases in aging human blastocysts. However, no-age-dependent changes in expression of the CXCR2, the cognate receptor for CXCL5, were found. In aging mice, Cxcl5 transcript levels were also increased in oocytes and embryos. Treatment of young mouse embryos with CXCL5 decreased implantation rates, together with increased expression of aging markers (P53, P21, Pai-1, and Il-6). Moreover, CXCL5 treatment suppressed trophoblast outgrowth in young mouse blastocysts. Conversely, suppression of CXCL5-CXCR2 signaling in aging mouse embryos using neutralizing antibodies and a receptor antagonist improved the implantation rate, leading to increases in pregnancy and delivery of normal pups. The gene expression pattern of these embryos was comparable to that in young mouse embryos showing enriched cell proliferation-related pathways. In conclusion, we identified CXCL5 as a SASP factor in human and mouse embryos and suppression of CXCL5-CXCR2 signaling during embryo culture improved pregnancy success in aging mice. Future analysis on CXCL5-CXCR2 signaling suppression in human embryos could be the basis to improve embryo development and pregnancy outcome in middle-aged infertile patients.
Keywords: CXCL5; CXCR2; SASP; aging; infertility; preimplantation embryo.
© 2020 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that they have no conflict of interest.
Figures


References
-
- Arenberg, D. A. , Keane, M. P. , DiGiovine, B. , Kunkel, S. L. , Morris, S. B. , Xue, Y. Y. , … Strieter, R. M. (1998). Epithelial‐neutrophil activating peptide (ENA‐78) is an important angiogenic factor in non‐small cell lung cancer. Journal of Clinical Investigation, 102(3), 465–472. 10.1172/jci3145 - DOI - PMC - PubMed
-
- Balamayooran, G. , Batra, S. , Cai, S. , Mei, J. , Worthen, G. S. , Penn, A. L. , & Jeyaseelan, S. (2012). Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. American Journal of Respiratory Cell and Molecular Biology, 47(1), 104–111. 10.1165/rcmb.2011-0260OC - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

