Shrinking body sizes in response to warming: explanations for the temperature-size rule with special emphasis on the role of oxygen
- PMID: 32959989
- PMCID: PMC7821163
- DOI: 10.1111/brv.12653
Shrinking body sizes in response to warming: explanations for the temperature-size rule with special emphasis on the role of oxygen
Abstract
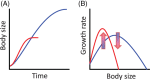
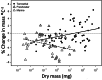
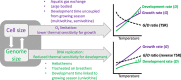
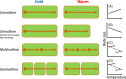
Body size is central to ecology at levels ranging from organismal fecundity to the functioning of communities and ecosystems. Understanding temperature-induced variations in body size is therefore of fundamental and applied interest, yet thermal responses of body size remain poorly understood. Temperature-size (T-S) responses tend to be negative (e.g. smaller body size at maturity when reared under warmer conditions), which has been termed the temperature-size rule (TSR). Explanations emphasize either physiological mechanisms (e.g. limitation of oxygen or other resources and temperature-dependent resource allocation) or the adaptive value of either a large body size (e.g. to increase fecundity) or a short development time (e.g. in response to increased mortality in warm conditions). Oxygen limitation could act as a proximate factor, but we suggest it more likely constitutes a selective pressure to reduce body size in the warm: risks of oxygen limitation will be reduced as a consequence of evolution eliminating genotypes more prone to oxygen limitation. Thus, T-S responses can be explained by the 'Ghost of Oxygen-limitation Past', whereby the resulting (evolved) T-S responses safeguard sufficient oxygen provisioning under warmer conditions, reflecting the balance between oxygen supply and demands experienced by ancestors. T-S responses vary considerably across species, but some of this variation is predictable. Body-size reductions with warming are stronger in aquatic taxa than in terrestrial taxa. We discuss whether larger aquatic taxa may especially face greater risks of oxygen limitation as they grow, which may be manifested at the cellular level, the level of the gills and the whole-organism level. In contrast to aquatic species, terrestrial ectotherms may be less prone to oxygen limitation and prioritize early maturity over large size, likely because overwintering is more challenging, with concomitant stronger end-of season time constraints. Mechanisms related to time constraints and oxygen limitation are not mutually exclusive explanations for the TSR. Rather, these and other mechanisms may operate in tandem. But their relative importance may vary depending on the ecology and physiology of the species in question, explaining not only the general tendency of negative T-S responses but also variation in T-S responses among animals differing in mode of respiration (e.g. water breathers versus air breathers), genome size, voltinism and thermally associated behaviour (e.g. heliotherms).
Keywords: Bergmann's rule; cell size; climate warming; gigantism; growth trajectory; hypoxia; life-history trade-off; phenotypic plasticity; temperature-size rule; thermal reaction norms.
© 2020 The Authors. Biological Reviews published by John Wiley & Sons Ltd on behalf of Cambridge Philosophical Society.
Figures

References
-
- Abrams, P. A. , Leimar, O. , Nylin, S. & Wiklund, C. (1996). The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. The American Naturalist 147, 381–395.
-
- Aguilar‐Alberola, J. A. & Mesquita‐Joanes, F. (2014). Breaking the temperature‐size rule: thermal effects on growth, development and fecundity of a crustacean from temporary waters. Journal of Thermal Biology 42, 15–24. - PubMed
-
- Angilletta, M. J. & Dunham, A. E. (2003). The temperature‐size rule in ectotherms: simple evolutionary explanations may not be general. The American Naturalist 162, 332–342. - PubMed
-
- Angilletta, M. J. , Steury, T. D. & Sears, M. W. (2004). Temperature, growth rate, and body size in ectotherms: fitting pieces of a life‐history puzzle. Integrative and Comparative Biology 44, 498–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

