Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature
- PMID: 32959998
- PMCID: PMC7607145
- DOI: 10.1002/ehf2.12958
Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature
Abstract
Aims: Cardiovascular complications, including myocarditis, are observed in coronavirus disease 2019 (COVID-19). Major cardiac involvement is a potentially lethal feature in severe cases. We sought to describe the underlying pathophysiological mechanism in COVID-19 lethal cardiogenic shock.
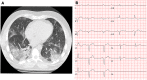
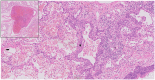
Methods and results: We report on a 48-year-old male COVID-19 patient with cardiogenic shock; despite extracorporeal life support, dialysis, and massive pharmacological support, this rescue therapy was not successful. Severe acute respiratory syndrome coronavirus 2 RNA was detected at autopsy in the lungs and myocardium. Histopathological examination revealed diffuse alveolar damage, proliferation of type II pneumocytes, lymphocytes in the lung interstitium, and pulmonary microemboli. Moreover, patchy muscular, sometimes perivascular, interstitial mononuclear inflammatory infiltrates, dominated by lymphocytes, were seen in the cardiac tissue. The lymphocytes 'interlocked' the myocytes, resulting in myocyte degeneration and necrosis. Predominantly, T-cell lymphocytes with a CD4:CD8 ratio of 1.7 infiltrated the interstitial myocardium, reflecting true myocarditis. The myocardial tissue was examined for markers of ferroptosis, an iron-catalysed form of regulated cell death that occurs through excessive peroxidation of polyunsaturated fatty acids. Immunohistochemical staining with E06, a monoclonal antibody binding to oxidized phosphatidylcholine (reflecting lipid peroxidation during ferroptosis), was positive in morphologically degenerating and necrotic cardiomyocytes adjacent to the infiltrate of lymphocytes, near arteries, in the epicardium and myocardium. A similar ferroptosis signature was present in the myocardium of a COVID-19 subject without myocarditis. In a case of sudden death due to viral myocarditis of unknown aetiology, however, immunohistochemical staining with E06 was negative. The renal proximal tubuli stained positively for E06 and also hydroxynonenal (4-HNE), a reactive breakdown product of the lipid peroxides that execute ferroptosis. In the case of myocarditis of other aetiology, the renal tissue displayed no positivity for E06 or 4-HNE.
Conclusions: The findings in this case are unique as this is the first report on accumulated oxidized phospholipids (or their breakdown products) in myocardial and renal tissue in COVID-19. This highlights ferroptosis, proposed to detrimentally contribute to some forms of ischaemia-reperfusion injury, as a detrimental factor in COVID-19 cardiac damage and multiple organ failure.
Keywords: Autopsy; COVID-19; Ferroptosis; Lymphocytic myocarditis; Renal failure; SARS-CoV-2-infection.
© 2020 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of the European Society of Cardiology.
Conflict of interest statement
The findings and conclusions in this report are those of the authors. The authors have nothing to disclose.
Figures




References
-
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DKW, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DGJC, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MPG, Drosten C. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill 2020; 25: 2000045. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
