Identification and Development of a New Positron Emission Tomography Ligand 4-(2-Fluoro-4-[11C]methoxyphenyl)-5-((1-methyl-1 H-pyrazol-3-yl)methoxy)picolinamide for Imaging Metabotropic Glutamate Receptor Subtype 2 (mGlu2)
- PMID: 32960052
- PMCID: PMC7892210
- DOI: 10.1021/acs.jmedchem.9b01991
Identification and Development of a New Positron Emission Tomography Ligand 4-(2-Fluoro-4-[11C]methoxyphenyl)-5-((1-methyl-1 H-pyrazol-3-yl)methoxy)picolinamide for Imaging Metabotropic Glutamate Receptor Subtype 2 (mGlu2)
Abstract
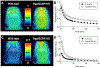
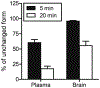
Metabotropic glutamate receptor 2 (mGlu2) is a known target for treating several central nervous system (CNS) disorders. To develop a viable positron emission tomography (PET) ligand for mGlu2, we identified new candidates 5a-i that are potent negative allosteric modulators (NAMs) of mGlu2. Among these candidates, 4-(2-fluoro-4-methoxyphenyl)-5-((1-methyl-1H-pyrazol-3-yl)methoxy)picolinamide (5i, also named as [11C]MG2-1812) exhibited high potency, high subtype selectivity, and favorable lipophilicity. Compound 5i was labeled with positron-emitting carbon-11 (11C) to obtain [11C]5i in high radiochemical yield and high molar activity by O-[11C]methylation of the phenol precursor 12 with [11C]CH3I. In vitro autoradiography with [11C]5i showed heterogeneous radioactive accumulation in the brain tissue sections, ranked in the order: cortex > striatum > hippocampus > cerebellum ≫ thalamus > pons. PET study of [11C]5i indicated in vivo specific binding of mGlu2 in the rat brain. Based on the [11C]5i scaffold, further optimization for new candidates is underway to identify a more suitable ligand for imaging mGlu2.
Figures

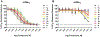







Similar articles
-
Synthesis and preliminary studies of 11C-labeled tetrahydro-1,7-naphthyridine-2-carboxamides for PET imaging of metabotropic glutamate receptor 2.Theranostics. 2020 Sep 14;10(24):11178-11196. doi: 10.7150/thno.42587. eCollection 2020. Theranostics. 2020. PMID: 33042277 Free PMC article.
-
Synthesis and Preliminary Studies of a Novel Negative Allosteric Modulator, 7-((2,5-Dioxopyrrolidin-1-yl)methyl)-4-(2-fluoro-4-[11C]methoxyphenyl) quinoline-2-carboxamide, for Imaging of Metabotropic Glutamate Receptor 2.ACS Chem Neurosci. 2017 Sep 20;8(9):1937-1948. doi: 10.1021/acschemneuro.7b00098. Epub 2017 Jun 13. ACS Chem Neurosci. 2017. PMID: 28565908 Free PMC article.
-
Synthesis and evaluation of 4-(2-fluoro-4-[11C]methoxyphenyl)-5-((2-methylpyridin-4-yl)methoxy)picolinamide for PET imaging of the metabotropic glutamate receptor 2 in the rat brain.Bioorg Med Chem. 2019 Feb 1;27(3):483-491. doi: 10.1016/j.bmc.2018.12.025. Epub 2018 Dec 17. Bioorg Med Chem. 2019. PMID: 30611634
-
Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2016 Dec;111:253-265. doi: 10.1016/j.neuropharm.2016.08.032. Epub 2016 Aug 30. Neuropharmacology. 2016. PMID: 27590915 Review.
-
Allosteric modulators of metabotropic glutamate receptors: lessons learnt from mGlu1, mGlu2 and mGlu5 potentiators and antagonists.Biochem Soc Trans. 2004 Nov;32(Pt 5):881-7. doi: 10.1042/BST0320881. Biochem Soc Trans. 2004. PMID: 15494040 Review.
Cited by
-
Upregulation of Striatal Metabotropic Glutamate Receptor Subtype 1 (mGluR1) in Rats with Excessive Glutamate Release Induced by N-Acetylcysteine.Neurotox Res. 2022 Feb;40(1):26-35. doi: 10.1007/s12640-021-00449-4. Epub 2022 Jan 4. Neurotox Res. 2022. PMID: 34981453
-
Radiosynthesis and Evaluation of 11C-Labeled Isoindolone-Based Positive Allosteric Modulators for Positron Emission Tomography Imaging of Metabotropic Glutamate Receptor 2.ACS Pharmacol Transl Sci. 2024 Jul 10;7(8):2414-2423. doi: 10.1021/acsptsci.4c00261. eCollection 2024 Aug 9. ACS Pharmacol Transl Sci. 2024. PMID: 39144551 Free PMC article.
-
Synthesis and Characterization of 5-(2-Fluoro-4-[11C]methoxyphenyl)-2,2-dimethyl-3,4-dihydro-2H-pyrano[2,3-b]pyridine-7-carboxamide as a PET Imaging Ligand for Metabotropic Glutamate Receptor 2.J Med Chem. 2022 Feb 10;65(3):2593-2609. doi: 10.1021/acs.jmedchem.1c02004. Epub 2022 Jan 28. J Med Chem. 2022. PMID: 35089713 Free PMC article.
References
-
- Kew JN; Kemp JA Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005, 179, 4–29. - PubMed
-
- Mukherjee S; Manahan-Vaughan D Role of metabotropic glutamate receptors in persistent forms of hippocampal plasticity and learning. Neuropharmacology 2013, 66, 65–81. - PubMed
-
- Schoepp DD Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 2001, 299, 12–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

