Comparative Safety and Efficacy of Treatments for Overactive Bladder Among Older Adults: A Network Meta-analysis
- PMID: 32960422
- PMCID: PMC7595992
- DOI: 10.1007/s40266-020-00792-9
Comparative Safety and Efficacy of Treatments for Overactive Bladder Among Older Adults: A Network Meta-analysis
Abstract
Background: Cumulative exposure to one or more anticholinergic medications ("anticholinergic burden") is associated with an increased risk of adverse outcomes, particularly among older individuals. Mirabegron, an oral selective β3-adrenergic receptor agonist, has demonstrated efficacy in managing the symptoms of overactive bladder without contributing to anticholinergic burden. However, it is not known whether the favorable safety profile of mirabegron relative to antimuscarinics varies with increasing age among a patient population who may have a high anticholinergic burden.
Objective: The primary objective of this study was to indirectly compare the safety and efficacy profile of mirabegron relative to antimuscarinics in older adults with overactive bladder.
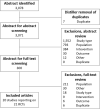
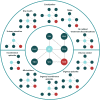
Methods: A systematic literature review was conducted to identify randomized controlled trials that reported safety and efficacy endpoints among patients aged ≥ 65 years. Identified randomized controlled trials were subsequently synthesized via a network meta-analysis. Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines in designing, performing, and reporting the literature review were followed. In line with current best practices, the network meta-analysis was conducted using a Bayesian approach and according to the overall general guidance for evidence synthesis developed by the National Institute for Health and Care Excellence decision support unit. Estimates of relative safety were assessed via the odds ratio and estimates of relative efficacy were assessed via means and credible intervals.
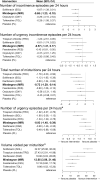
Results: A total of 3078 abstracts, 300 of which underwent full-text screening, were identified using the search criteria. Twenty articles reporting on 21 randomized controlled trials were eligible for data extraction and synthesis. Following review, five safety and five efficacy endpoints were considered for inclusion in the network meta-analysis. Regarding findings typical of anticholinergic exposure in older adults, mirabegron was not associated with an increased odds of dry mouth (odds ratio 95% credible interval 0.76 [0.26-2.37]) or constipation (1.08 [0.39-3.02]) relative to placebo, whereas antimuscarinics were strongly associated with these events (odds ratio range 3.78-7.85 and 2.12-4.66, respectively). In this older population, mirabegron was associated with a similar odds of experiencing adverse event-related treatment discontinuations relative to placebo (0.99 [0.57-1.70]), while the odds of experiencing an adverse event-related treatment discontinuation for antimuscarinics had a range of 1.14-3.03 (in most cases, the association was mild). No increased odds of experiencing overall treatment-emergent adverse events was observed for mirabegron or antimuscarinics (odds ratio range 1.25-1.55), apart from fesoterodine (2.23 [1.37-3.37]). Finally, a similar treatment effect was observed across all efficacy endpoints between mirabegron and antimuscarinics in this older population.
Conclusions: This study indicates that the safety and efficacy profile of mirabegron remains favorable compared with antimuscarinics among older adults. This includes safety outcomes typically associated with anticholinergic burden, which were less frequently observed in patients treated with mirabegron.
Conflict of interest statement
David Walker, Rita Kristy, John Hairston, and Carol Schermer are employees of Astellas Pharma Global Development, Inc., which sponsored the study. Greta Lozano-Ortega, Karissa Johnston, Basia Rogula, Alexis Mickle, and Sean Harrigan have no conflicts of interest that are directly relevant to the content of this study. Broadstreet Health Economics and Outcomes Research received funding from Astellas Pharma Global Development, Inc. to assist in the development, conduct, and analysis of the study.
Figures

Comment in
-
Re: Comparative Safety and Efficacy of Treatments for Overactive Bladder Among Older Adults: A Network Meta-analysis.Eur Urol. 2022 Feb;81(2):214. doi: 10.1016/j.eururo.2021.11.004. Epub 2021 Nov 20. Eur Urol. 2022. PMID: 34810012 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

