Sex Representation in Clinical Trials Associated with FDA Cancer Drug Approvals Differs Between Solid and Hematologic Malignancies
- PMID: 32960478
- PMCID: PMC7873318
- DOI: 10.1002/onco.13534
Sex Representation in Clinical Trials Associated with FDA Cancer Drug Approvals Differs Between Solid and Hematologic Malignancies
Abstract
Background: Proportionate female representation in health research is necessary for scientific rigor and health equity. We aimed to assess the representation of women in clinical trials leading to U.S. Food and Drug Administration (FDA) cancer drug approvals.
Materials and methods: Trials supporting FDA cancer drug approvals between July 2008 and June 2018 were sourced from PubMed and ClinicalTrials.gov. The ratio of female to male trial enrollment was compared with cancer incidence and mortality in the U.S. using International Agency for Research on Cancer data. Reproductive tract and breast cancers were excluded. Odds ratios (ORs) and 95% confidence intervals (CIs) comparing trial enrollment with population incidence and mortality were calculated.
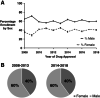
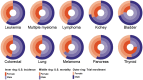
Results: A total of 186 trials leading to 170 FDA cancer drug approvals showed slight female underrepresentation compared with overall cancer incidence in the U.S. (OR, 0.97; 95% CI, 0.95-0.98, p < .0001). Female enrollment for drugs approved between 2008-2013 and 2014-2018 was unchanged (OR, 1.02; 95% CI, 0.99-1.05, p = .25). There was slight female underrepresentation in hematological trials (OR, 0.95; 95% CI, 0.91-0.998; p = .040 for leukemia; OR, 0.95; 95% CI, 0.90-0.997; p = .040 for lymphoma) and significant female underrepresentation in colorectal (OR, 0.72; 95% CI, 0.69-0.76; p < .0001), pancreas (OR, 0.85; 95% CI, 0.78-0.93; p = .0004), lung (OR, 0.77; 95% CI, 0.75-0.80; p < .0001), kidney (OR, 0.63; 95% CI, 0.60-0.67; p < .0001), and thyroid cancer trials (OR, 0.26; 95% CI, 0.23-0.28; p < .0001) compared with U.S. incidence.
Conclusion: Female underrepresentation has persisted within solid organ tumor trials but is less notable in hematologic trials. Additional work is required to identify drivers of such disparity.
Implications for practice: Adequate gender representation in clinical trials is a matter of health equity. This study demonstrates that women remain underrepresented in trials across hematological and solid organ trials compared with cancer incidence and mortality in women, with the disparity worse in a number of solid organ tumor types. There are thus still significant improvements to be made regarding adequate representation of women in trials. Studies exploring the reasons for ongoing disparity in gender representation are warranted to help clinicians to rectify this.
Keywords: Clinical trial; Healthcare disparities; Neoplasms; Sex.
© AlphaMed Press 2020.
Conflict of interest statement
Figures

Similar articles
-
Assessment of gender representation in clinical trials leading to FDA approval for oncology therapeutics between 2014 and 2019: A systematic review-based cohort study.Cancer. 2021 Sep 1;127(17):3156-3162. doi: 10.1002/cncr.33533. Epub 2021 Jun 23. Cancer. 2021. PMID: 34160824
-
Racial/Ethnic Disparities in Ophthalmology Clinical Trials Resulting in US Food and Drug Administration Drug Approvals From 2000 to 2020.JAMA Ophthalmol. 2021 Jun 1;139(6):629-637. doi: 10.1001/jamaophthalmol.2021.0857. JAMA Ophthalmol. 2021. PMID: 33885724 Free PMC article.
-
Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018.JAMA Oncol. 2019 Oct 1;5(10):e191870. doi: 10.1001/jamaoncol.2019.1870. Epub 2019 Oct 10. JAMA Oncol. 2019. PMID: 31415071 Free PMC article.
-
Impact of Global Enrollment on Race, Ethnicity, and Age Representation in Pivotal Gynecologic Cancer Trials Leading to US Food and Drug Administration Drug Approvals.JCO Glob Oncol. 2025 May;11:e2400636. doi: 10.1200/GO-24-00636. Epub 2025 May 29. JCO Glob Oncol. 2025. PMID: 40440574 Free PMC article.
-
Older adults in hematologic malignancy trials: Representation, barriers to participation and strategies for addressing underrepresentation.Blood Rev. 2020 Sep;43:100670. doi: 10.1016/j.blre.2020.100670. Epub 2020 Feb 7. Blood Rev. 2020. PMID: 32241586 Review.
Cited by
-
Inclusive oncological trials and targeted treatments cannot ignore sex and gender.BMJ Oncol. 2023 Jun 20;2(1):e000051. doi: 10.1136/bmjonc-2023-000051. eCollection 2023. BMJ Oncol. 2023. PMID: 39886518 Free PMC article. No abstract available.
-
Kidney function and cancer risk: An analysis using creatinine and cystatin C in a cohort study.EClinicalMedicine. 2021 Jul 26;38:101030. doi: 10.1016/j.eclinm.2021.101030. eCollection 2021 Aug. EClinicalMedicine. 2021. PMID: 34505030 Free PMC article.
-
Analysis of Female Participant Representation in Registered Oncology Clinical Trials in the United States from 2008 to 2020.Oncologist. 2023 Jun 2;28(6):510-519. doi: 10.1093/oncolo/oyad009. Oncologist. 2023. PMID: 36848266 Free PMC article.
-
Pitfalls and Successes in Trials in Older Transplant Patients with Hematologic Malignancies.Curr Oncol Rep. 2022 Jan;24(1):125-133. doi: 10.1007/s11912-022-01194-3. Epub 2022 Jan 21. Curr Oncol Rep. 2022. PMID: 35061190 Review.
-
Sex-Related Differences in Immunotherapy Toxicities: Insights into Dimorphic Responses.Cancers (Basel). 2025 Mar 21;17(7):1054. doi: 10.3390/cancers17071054. Cancers (Basel). 2025. PMID: 40227458 Free PMC article. Review.
References
-
- U.S. Department of Health and Human Services, Food and Drug Administration . General Considerations for the Clinical Evaluation of Drugs. Rockville, MD: U.S. Food and Drug Administration; 1977.
-
- U.S. Congress Public Law. National Institutes of Health Revitalization Act of 1993: Act to Amend the Public Health Service Act to Revise and Extend the Programs of the National Institutes of Health, and for Other Purposes, S. 1, 103rd Cong (1993).
-
- Investigational new drug applications ; Amendment to clinical hold regulations for products intended for life‐threatening diseases and conditions. Food and Drug Administration, HHS. Final rule. Fed Regist 2000;65:34963–34971. - PubMed
-
- U.S. Government Accountability Office . GAO‐01‐286R: Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women. Washington, DC: U.S. Government Accountability Office; 2001.
-
- Investigational new drug applications and new drug applications–FDA . Final rule. Fed Regist 1998;63:6854–6862. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

