Antibody screening using a human iPSC-based blood-brain barrier model identifies antibodies that accumulate in the CNS
- PMID: 32960493
- PMCID: PMC7513543
- DOI: 10.1096/fj.202000851R
Antibody screening using a human iPSC-based blood-brain barrier model identifies antibodies that accumulate in the CNS
Abstract
Drug delivery across the blood-brain barrier (BBB) remains a significant obstacle for the development of neurological disease therapies. The low penetration of blood-borne therapeutics into the brain can oftentimes be attributed to the restrictive nature of the brain microvascular endothelial cells (BMECs) that comprise the BBB. One strategy beginning to be successfully leveraged is the use of endogenous receptor-mediated transcytosis (RMT) systems as a means to shuttle a targeted therapeutic into the brain. Limitations of known RMT targets and their cognate targeting reagents include brain specificity, brain uptake levels, and off-target effects, driving the search for new and potentially improved brain targeting reagent-RMT pairs. To this end, we deployed human-induced pluripotent stem cell (iPSC)-derived BMEC-like cells as a model BBB substrate on which to mine for new RMT-targeting antibody pairs. A nonimmune, human single-chain variable fragment (scFv) phage display library was screened for binding, internalization, and transcytosis across iPSC-derived BMECs. Lead candidates exhibited binding and internalization into BMECs as well as binding to both human and mouse BBB in brain tissue sections. Antibodies targeted the murine BBB after intravenous administration with one particular clone, 46.1-scFv, exhibiting a 26-fold increase in brain accumulation (8.1 nM). Moreover, clone 46.1-scFv was found to associate with postvascular, parenchymal cells, indicating its successful receptor-mediated transport across the BBB. Such a new BBB targeting ligand could enhance the transport of therapeutic molecules into the brain.
Keywords: antibody‐mediated brain drug delivery; blood‐brain barrier; nicotinamide adenine dinucleotide.
© 2020 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Competing interests:
J.V.G., L.I.G. C.C.S and E.V.S. are inventors on a provisional US patent application.
Figures

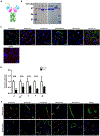
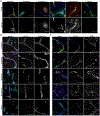

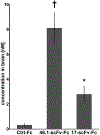
Similar articles
-
A Perfused In Vitro Human iPSC-Derived Blood-Brain Barrier Faithfully Mimics Transferrin Receptor-Mediated Transcytosis of Therapeutic Antibodies.Cell Mol Neurobiol. 2023 Nov;43(8):4173-4187. doi: 10.1007/s10571-023-01404-x. Epub 2023 Sep 12. Cell Mol Neurobiol. 2023. PMID: 37698826 Free PMC article.
-
Mouse embryonic stem cell-derived blood-brain barrier model: applicability to studying antibody triggered receptor mediated transcytosis.Fluids Barriers CNS. 2023 May 26;20(1):36. doi: 10.1186/s12987-023-00437-0. Fluids Barriers CNS. 2023. PMID: 37237379 Free PMC article.
-
Strategies to identify, engineer, and validate antibodies targeting blood-brain barrier receptor-mediated transcytosis systems for CNS drug delivery.Expert Opin Drug Deliv. 2023 Jul-Dec;20(12):1789-1800. doi: 10.1080/17425247.2023.2286371. Epub 2023 Dec 29. Expert Opin Drug Deliv. 2023. PMID: 38007619 Free PMC article. Review.
-
Receptor-mediated transcytosis of nanobodies targeting the heparin-binding EGF-like growth factor in human blood-brain barrier models.J Control Release. 2025 Jul 10;383:113852. doi: 10.1016/j.jconrel.2025.113852. Epub 2025 May 18. J Control Release. 2025. PMID: 40393531
-
Receptor-mediated transcytosis of macromolecules across the blood-brain barrier.Expert Opin Drug Deliv. 2023 Jul-Dec;20(12):1699-1711. doi: 10.1080/17425247.2023.2255138. Epub 2023 Sep 15. Expert Opin Drug Deliv. 2023. PMID: 37658673 Review.
Cited by
-
Enhanced in Vivo Blood Brain Barrier Transcytosis of Macromolecular Cargo Using an Engineered pH-sensitive Mouse Transferrin Receptor Binding Nanobody.bioRxiv [Preprint]. 2023 Apr 27:2023.04.26.538462. doi: 10.1101/2023.04.26.538462. bioRxiv. 2023. Update in: Fluids Barriers CNS. 2023 Aug 24;20(1):64. doi: 10.1186/s12987-023-00462-z. PMID: 37333358 Free PMC article. Updated. Preprint.
-
A Pralidoxime Nanocomplex Formulation Targeting Transferrin Receptors for Reactivation of Brain Acetylcholinesterase After Exposure of Mice to an Anticholinesterase Organophosphate.Int J Nanomedicine. 2024 Jan 12;19:307-326. doi: 10.2147/IJN.S443498. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38229703 Free PMC article.
-
Identification of lamprey variable lymphocyte receptors that target the brain vasculature.Sci Rep. 2022 Apr 11;12(1):6044. doi: 10.1038/s41598-022-09962-8. Sci Rep. 2022. PMID: 35411012 Free PMC article.
-
An innovative strategy to identify new targets for delivering antibodies to the brain has led to the exploration of the integrin family.PLoS One. 2022 Sep 15;17(9):e0274667. doi: 10.1371/journal.pone.0274667. eCollection 2022. PLoS One. 2022. PMID: 36108060 Free PMC article.
-
Device-assisted strategies for drug delivery across the blood-brain barrier to treat glioblastoma.Commun Mater. 2025;6(1):5. doi: 10.1038/s43246-024-00721-y. Epub 2025 Jan 7. Commun Mater. 2025. PMID: 39790893 Free PMC article. Review.
References
-
- Pardridge WM, Kang YS, and Buciak JL (1994) Transport of human recombinant brain-derived neurotrophic factor ({BDNF}) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm. Res 11, 738–746 - PubMed
-
- Pardridge WM, Kang YS, Buciak JL, and Yang J (1995) Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood-brain barrier in vivo in the primate. Pharm. Res 12, 807–816 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

