Identification of novel candidate risk genes for myelomeningocele within the glucose homeostasis/oxidative stress and folate/one-carbon metabolism networks
- PMID: 32960507
- PMCID: PMC7667334
- DOI: 10.1002/mgg3.1495
Identification of novel candidate risk genes for myelomeningocele within the glucose homeostasis/oxidative stress and folate/one-carbon metabolism networks
Abstract
Background: Neural tube defects (NTDs) are the second most common complex birth defect, yet, our understanding of the genetic contribution to their development remains incomplete. Two environmental factors associated with NTDs are Folate and One Carbon Metabolism (FOCM) and Glucose Homeostasis and Oxidative Stress (GHOS). Utilizing next-generation sequencing of a large patient cohort, we identify novel candidate genes in these two networks to provide insights into NTD mechanisms.
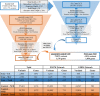
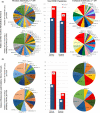
Methods: Exome sequencing (ES) was performed in 511 patients, born with myelomeningocele, divided between European American and Mexican American ethnicities. Healthy control data from the Genome Aggregation database were ethnically matched and used as controls. Rare, high fidelity, nonsynonymous predicted damaging missense, nonsense, or canonical splice site variants in independently generated candidate gene lists for FOCM and GHOS were identified. We used a gene-based collapsing approach to quantify mutational burden in case and controls, with the control cohort estimated using cumulative allele frequencies assuming Hardy-Weinberg equilibrium.
Results: We identified 45 of 837 genes in the FOCM network and 22 of 568 genes in the GHOS network as possible NTD risk genes with p < 0.05. No nominally significant risk genes were shared between ethnicities. Using a novel approach to mutational burden we identify 55 novel NTD risk associations.
Conclusions: We provide a means of utilizing large publicly available sequencing datasets as controls for sequencing projects examining rare disease. This approach confirmed existing risk genes for myelomeningocele and identified possible novel risk genes. Lastly, it suggests possible distinct genetic etiologies for this malformation between different ethnicities.
Keywords: Myelomeningocele; exome sequencing; folate; glucose; mutation burden.
© 2020 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals LLC.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


Similar articles
-
Mutations in folate transporter genes and risk for human myelomeningocele.Am J Med Genet A. 2017 Nov;173(11):2973-2984. doi: 10.1002/ajmg.a.38472. Epub 2017 Sep 26. Am J Med Genet A. 2017. PMID: 28948692 Free PMC article.
-
Genetic studies of myelomeningocele.Childs Nerv Syst. 2013 Sep;29(9):1417-25. doi: 10.1007/s00381-013-2197-2. Epub 2013 Sep 7. Childs Nerv Syst. 2013. PMID: 24013315 Review.
-
Burden of rare deleterious variants in WNT signaling genes among 511 myelomeningocele patients.PLoS One. 2020 Sep 24;15(9):e0239083. doi: 10.1371/journal.pone.0239083. eCollection 2020. PLoS One. 2020. PMID: 32970752 Free PMC article.
-
Polymorphisms in maternal folate pathway genes interact with arsenic in drinking water to influence risk of myelomeningocele.Birth Defects Res A Clin Mol Teratol. 2015 Sep;103(9):754-62. doi: 10.1002/bdra.23399. Epub 2015 Aug 6. Birth Defects Res A Clin Mol Teratol. 2015. PMID: 26250961 Free PMC article.
-
Spina bifida and other neural tube defects.Curr Probl Pediatr. 2000 Nov-Dec;30(10):313-32. doi: 10.1067/mpp.2000.112052. Curr Probl Pediatr. 2000. PMID: 11147289 Review.
Cited by
-
Multifactorial Rare Diseases: Can Uncertainty Analysis Bring Added Value to the Search for Risk Factors and Etiopathogenesis?Medicina (Kaunas). 2021 Jan 28;57(2):119. doi: 10.3390/medicina57020119. Medicina (Kaunas). 2021. PMID: 33525390 Free PMC article. Review.
-
Spina bifida as a multifactorial birth defect: Risk factors and genetic underpinnings.Pediatr Discov. 2025 Jan 25;3(2):e2517. doi: 10.1002/pdi3.2517. eCollection 2025 Jun. Pediatr Discov. 2025. PMID: 40666233 Free PMC article. Review.
-
Unraveling the complex genetics of neural tube defects: From biological models to human genomics and back.Genesis. 2021 Nov;59(11):e23459. doi: 10.1002/dvg.23459. Epub 2021 Oct 29. Genesis. 2021. PMID: 34713546 Free PMC article. Review.
-
Opportunities and challenges for the use of common controls in sequencing studies.Nat Rev Genet. 2022 Nov;23(11):665-679. doi: 10.1038/s41576-022-00487-4. Epub 2022 May 17. Nat Rev Genet. 2022. PMID: 35581355 Free PMC article. Review.
-
Human myelomeningocele risk and ultra-rare deleterious variants in genes associated with cilium, WNT-signaling, ECM, cytoskeleton and cell migration.Sci Rep. 2021 Feb 11;11(1):3639. doi: 10.1038/s41598-021-83058-7. Sci Rep. 2021. PMID: 33574475 Free PMC article.
References
-
- Arth, A. C. , Tinker, S. C. , Simeone, R. M. , Ailes, E. C. , Cragan, J. D. , & Grosse, S. D. (2017). Inpatient hospitalization costs associated with birth defects among persons of all ages — United States, 2013. MMWR. Morbidity and Mortality Weekly Report, 66(02), 41–46. 10.15585/mmwr.mm6602a1 - DOI - PMC - PubMed
-
- Au, K. S. , Tran, P. X. , Tsai, C. C. , O’Byrne, M. R. , Lin, J. , Morrison, A. C. , … Northrup, H. (2008). Characteristics of a spina bifida population including North American Caucasian and Hispanic individuals. Birth Defects Research. Part A, Clinical and Molecular Teratology, 82(10), 692–700. 10.1002/bdra.20499 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

