Longitudinal monitoring of microglial/macrophage activation in ischemic rat brain using Iba-1-specific nanoparticle-enhanced magnetic resonance imaging
- PMID: 32960690
- PMCID: PMC7687035
- DOI: 10.1177/0271678X20953913
Longitudinal monitoring of microglial/macrophage activation in ischemic rat brain using Iba-1-specific nanoparticle-enhanced magnetic resonance imaging
Abstract
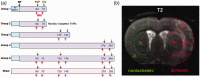
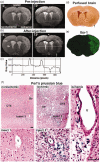
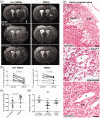
Microglial/macrophage activation plays a dual role in response to brain injury after a stroke, promoting early neuroinflammation and benefit for neurovascular recovery. Therefore, the dynamics of stroke-induced cerebral microglial/macrophage activation are of substantial interest. This study used novel anti-Iba-1-targeted superparamagnetic iron-platinum (FePt) nanoparticles in conjunction with magnetic resonance imaging (MRI) to measure the spatiotemporal changes of the microglial/macrophage activation in living rat brain for four weeks post-stroke. Ischemic lesion areas were identified and measured using T2-weighted MR images. After injection of the FePt-nanoparticles, T2*-weighted MR images showed that the nanoparticles were seen solely in brain regions that coincided with areas of active microglia/macrophages detected by post-mortem immunohistochemistry. Good agreement in morphological and distributive dynamic changes was also observed between the Fe+-cells and the Iba-1+-microglia/macrophages. The spatiotemporal changes of nanoparticle detected by T2*-weighted images paralleled the changes of microglial/macrophage activation and phenotypes measured by post-mortem immunohistochemistry over the four weeks post-stroke. Maximum microglial/macrophage activation occurred seven days post-stroke for both measures, and the diminished activation found after two weeks continued to four weeks. Our results suggest that nanoparticle-enhanced MRI may constitute a novel approach for monitoring the dynamic development of neuroinflammation in living animals during the progression and treatment of stroke.
Keywords: Iba-1-antibody-conjugated superparamagnetic iron–platinum nanoparticles; Ischemic stroke; magnetic resonance imaging; microglial/macrophage activation; neuroinflammation.
Conflict of interest statement
Figures




Similar articles
-
Post-stroke Intranasal (+)-Naloxone Delivery Reduces Microglial Activation and Improves Behavioral Recovery from Ischemic Injury.eNeuro. 2018 Apr 18;5(2):ENEURO.0395-17.2018. doi: 10.1523/ENEURO.0395-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29766045 Free PMC article.
-
Impact of hydroxytyrosol on stroke: tracking therapy response on neuroinflammation and cerebrovascular parameters using PET-MR imaging and on functional outcomes.Theranostics. 2021 Feb 15;11(9):4030-4049. doi: 10.7150/thno.48110. eCollection 2021. Theranostics. 2021. PMID: 33754046 Free PMC article.
-
Superparamagnetic nanoparticle-enhanced MRI of Alzheimer's disease plaques and activated microglia in 3X transgenic mouse brains: Contrast optimization.J Magn Reson Imaging. 2017 Aug;46(2):574-588. doi: 10.1002/jmri.25563. Epub 2016 Nov 22. J Magn Reson Imaging. 2017. PMID: 27875002
-
The dual function of microglial polarization and its treatment targets in ischemic stroke.Front Neurol. 2022 Sep 23;13:921705. doi: 10.3389/fneur.2022.921705. eCollection 2022. Front Neurol. 2022. PMID: 36212660 Free PMC article. Review.
-
Magnetic resonance imaging of human brain macrophage infiltration.Neurotherapeutics. 2007 Jul;4(3):434-42. doi: 10.1016/j.nurt.2007.05.005. Neurotherapeutics. 2007. PMID: 17599709 Free PMC article. Review.
Cited by
-
Revolutionizing Stroke Care: Nanotechnology-Based Brain Delivery as a Novel Paradigm for Treatment and Diagnosis.Mol Neurobiol. 2025 Jan;62(1):184-220. doi: 10.1007/s12035-024-04215-3. Epub 2024 Jun 3. Mol Neurobiol. 2025. PMID: 38829514 Review.
-
β-Sitosterol Alleviates Neuropathic Pain by Affect Microglia Polarization through Inhibiting TLR4/NF-κB Signaling Pathway.J Neuroimmune Pharmacol. 2023 Dec;18(4):690-703. doi: 10.1007/s11481-023-10091-w. Epub 2023 Dec 2. J Neuroimmune Pharmacol. 2023. PMID: 38041701
-
Neuroinflammation in the Evolution of Motor Function in Stroke and Trauma Patients: Treatment and Potential Biomarkers.Curr Issues Mol Biol. 2023 Oct 25;45(11):8552-8585. doi: 10.3390/cimb45110539. Curr Issues Mol Biol. 2023. PMID: 37998716 Free PMC article. Review.
-
Recent Advances in Nanomaterials for Diagnosis, Treatments, and Neurorestoration in Ischemic Stroke.Front Cell Neurosci. 2022 Jun 28;16:885190. doi: 10.3389/fncel.2022.885190. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35836741 Free PMC article. Review.
-
Current Status of Research on Nanomaterials Combined with Mesenchymal Stem Cells for the Treatment of Ischemic Stroke.Neuromolecular Med. 2024 Dec 7;26(1):51. doi: 10.1007/s12017-024-08819-9. Neuromolecular Med. 2024. PMID: 39644405 Review.
References
-
- Bai Q, Xue M, Yong VW. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: therapeutic opportunities. Brain 2020; 143:1297–1314. - PubMed
-
- Schwartz M, Deczkowska A. Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol 2016; 37: 668–679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

