Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data
- PMID: 32960899
- PMCID: PMC7508405
- DOI: 10.1371/journal.pone.0239401
Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data
Abstract
Objective: To analyze the effects of a short course of methyl-prednisolone pulses (MP) during the second week of disease (week-2) in patients with severe coronavirus disease 2019 (COVID-19) pneumonia.
Methods: Comparative observational study using data collected from routine care at Hospital Universitario Cruces, Barakaldo, Bizkaia, Spain in patients with COVID-19 pneumonia. We compared patients who received week-2-MP (125-250 mg/d x3) with those who did not, with the end-points time to death and time to death or endotracheal intubation.
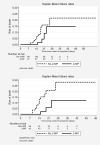
Results: We included 242 patients with COVID-19 pneumonia and elevated inflammatory markers at admission. Sixty-one patients (25%) received week-2-MP. Twenty-two patients (9%) died and 31 (12.8%) suffered death or intubation. The adjusted HRs for death and death or intubation for patients in the week-2-MP group were 0.35 (95%CI 0.11 to 1.06, p = 0.064) and 0.33 (95%CI 0.13 to 0.84, p = 0.020), respectively. These differences were specifically seen in the subcohort of patients with a SpO2/FiO2 at day 7 lower than 353 (adjusted HR 0.31, 95% CI 0.08 to 1.12, p = 0.073 and HR 0.34, 95%CI 0.12 to 0.94, p = 0.038, respectively) but not in patients with higher SpO2/FiO2. Patients receiving out-of-week-2-MP, non-pulse glucocorticoids or no glucocorticoids had an increased adjusted risk for both outcomes compared with week-2-MP group: HR 5.04 (95% CI 0.91-27.86), HR 10.09 (95% CI 2.14-47.50), HR 4.14 (95% CI 0.81-21.23), respectively, for death; HR 7.38 (95% CI 1.86-29.29), HR 13.71 (95% CI 3.76-50.07), HR 3.58 (95% CI 0.89-14.32), respectively, for death or intubation. These differences were significant only in the subgroup with low SpO2/FiO2.
Conclusions: Week-2-MP are effective in improving the prognosis of patients with COVID-19 pneumonia with features of inflammatory activity and respiratory deterioration entering the second week of disease. The recognition of this high-risk population should prompt early use of MP at this point.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (no) infection is suspected (WHO/2019-nCoV/clinical/2020.4). Updated 13 Mar 2020. https://www.who.int/publicatio ns-detail/clinical-management-of-severe- acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed 27 Mar 2020).
-
- Documento técnico. Manejo clínico del COVID-19: tratamiento médico. 19 de marzo de 2020. Ministerio de Sanidad, Gobierno de España. http://www.aeemt.com/web/wp-content/uploads/2020/03/4_602630019391212910.... Accessed 20 March 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

