Genomic Signatures of Coevolution between Nonmodel Mammals and Parasitic Roundworms
- PMID: 32960966
- PMCID: PMC7826172
- DOI: 10.1093/molbev/msaa243
Genomic Signatures of Coevolution between Nonmodel Mammals and Parasitic Roundworms
Abstract
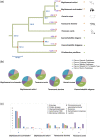
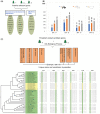
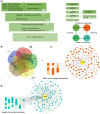
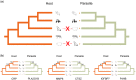
Antagonistic coevolution between host and parasite drives species evolution. However, most of the studies only focus on parasitism adaptation and do not explore the coevolution mechanisms from the perspective of both host and parasite. Here, through the de novo sequencing and assembly of the genomes of giant panda roundworm, red panda roundworm, and lion roundworm parasitic on tiger, we investigated the genomic mechanisms of coevolution between nonmodel mammals and their parasitic roundworms and those of roundworm parasitism in general. The genome-wide phylogeny revealed that these parasitic roundworms have not phylogenetically coevolved with their hosts. The CTSZ and prolyl 4-hydroxylase subunit beta (P4HB) immunoregulatory proteins played a central role in protein interaction between mammals and parasitic roundworms. The gene tree comparison identified that seven pairs of interactive proteins had consistent phylogenetic topology, suggesting their coevolution during host-parasite interaction. These coevolutionary proteins were particularly relevant to immune response. In addition, we found that the roundworms of both pandas exhibited higher proportions of metallopeptidase genes, and some positively selected genes were highly related to their larvae's fast development. Our findings provide novel insights into the genetic mechanisms of coevolution between nonmodel mammals and parasites and offer the valuable genomic resources for scientific ascariasis prevention in both pandas.
Keywords: coevolution; comparative genomics; pandas; parasitism.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures
Similar articles
-
Chromosome-scale assembly and whole-genome sequencing of 266 giant panda roundworms provide insights into their evolution, adaptation and potential drug targets.Mol Ecol Resour. 2022 Feb;22(2):768-785. doi: 10.1111/1755-0998.13504. Epub 2021 Oct 28. Mol Ecol Resour. 2022. PMID: 34549895 Free PMC article.
-
Genome of the Giant Panda Roundworm Illuminates Its Host Shift and Parasitic Adaptation.Genomics Proteomics Bioinformatics. 2022 Apr;20(2):366-381. doi: 10.1016/j.gpb.2021.08.002. Epub 2021 Sep 3. Genomics Proteomics Bioinformatics. 2022. PMID: 34487863 Free PMC article.
-
Major histocompatibility complex alleles associated with parasite susceptibility in wild giant pandas.Heredity (Edinb). 2015 Jan;114(1):85-93. doi: 10.1038/hdy.2014.73. Epub 2014 Sep 24. Heredity (Edinb). 2015. PMID: 25248466 Free PMC article.
-
Novel genomic approaches to study antagonistic coevolution between hosts and parasites.Mol Ecol. 2021 Aug;30(15):3660-3676. doi: 10.1111/mec.16001. Epub 2021 Jun 22. Mol Ecol. 2021. PMID: 34038012 Review.
-
The role of defensive symbionts in host-parasite coevolution.Biol Rev Camb Philos Soc. 2018 Nov;93(4):1747-1764. doi: 10.1111/brv.12417. Epub 2018 Apr 16. Biol Rev Camb Philos Soc. 2018. PMID: 29663622 Review.
Cited by
-
Parasitoid Serpins Evolve Novel Functions to Manipulate Host Homeostasis.Mol Biol Evol. 2023 Dec 1;40(12):msad269. doi: 10.1093/molbev/msad269. Mol Biol Evol. 2023. PMID: 38061001 Free PMC article.
-
Assessing de novo parasite genomes assembled using only Oxford Nanopore Technologies MinION data.iScience. 2024 Jul 30;27(9):110614. doi: 10.1016/j.isci.2024.110614. eCollection 2024 Sep 20. iScience. 2024. PMID: 39211578 Free PMC article.
-
Chromosome-scale assembly and whole-genome sequencing of 266 giant panda roundworms provide insights into their evolution, adaptation and potential drug targets.Mol Ecol Resour. 2022 Feb;22(2):768-785. doi: 10.1111/1755-0998.13504. Epub 2021 Oct 28. Mol Ecol Resour. 2022. PMID: 34549895 Free PMC article.
-
Getting around the roundworms: Identifying knowledge gaps and research priorities for the ascarids.Adv Parasitol. 2024;123:51-123. doi: 10.1016/bs.apar.2023.12.002. Epub 2024 Feb 20. Adv Parasitol. 2024. PMID: 38448148 Free PMC article.
-
Genomics of the Parasitic Nematode Ascaris and Its Relatives.Genes (Basel). 2021 Mar 28;12(4):493. doi: 10.3390/genes12040493. Genes (Basel). 2021. PMID: 33800545 Free PMC article. Review.
References
-
- Beames CG Jr, King GA.. 1972. Factors influencing the movement of materials across the intestine of Ascaris. In: Comparative biochemistry of parasites. New York: Academic Press. p. 275–282.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

