Site-Specific Incorporation of a Photoactivatable Fluorescent Amino Acid
- PMID: 32961013
- PMCID: PMC8011588
- DOI: 10.1002/cbic.202000602
Site-Specific Incorporation of a Photoactivatable Fluorescent Amino Acid
Abstract
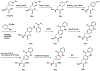
Photoactivatable fluorophores are emerging optical probes for biological applications. Most photoactivatable fluorophores are relatively large in size and need to be activated by ultraviolet light; this dramatically limits their applications. To introduce photoactivatable fluorophores into proteins, recent investigations have explored several protein-labeling technologies, including fluorescein arsenical hairpin (FlAsH) Tag, HaloTag labeling, SNAPTag labeling, and other bioorthogonal chemistry-based methods. However, these technologies require a multistep labeling process. Here, by using genetic code expansion and a single sulfur-for-oxygen atom replacement within an existing fluorescent amino acid, we have site-specifically incorporated the photoactivatable fluorescent amino acid thioacridonylalanine (SAcd) into proteins in a single step. Moreover, upon exposure to visible light, SAcd can be efficiently desulfurized to its oxo derivatives, thus restoring the strong fluorescence of labeled proteins.
Keywords: genetic code expansion; noncanonical amino acids; optical probes; photoactivatable fluorophores; protein labeling.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures


Similar articles
-
Sulfur-tetrazine as highly efficient visible-light activatable photo-trigger for designing photoactivatable fluorescence biomolecules.J Mater Chem B. 2024 Oct 30;12(42):10839-10849. doi: 10.1039/d4tb01817f. J Mater Chem B. 2024. PMID: 39420843
-
Single-Atom Fluorescence Switch: A General Approach toward Visible-Light-Activated Dyes for Biological Imaging.J Am Chem Soc. 2019 Sep 18;141(37):14699-14706. doi: 10.1021/jacs.9b06237. Epub 2019 Sep 9. J Am Chem Soc. 2019. PMID: 31450884 Free PMC article.
-
Photoactivatable fluorophores for durable labelling of individual cells.Chem Commun (Camb). 2021 Jun 10;57(47):5802-5805. doi: 10.1039/d1cc01488a. Chem Commun (Camb). 2021. PMID: 33999073
-
Photostable and photoswitching fluorescent dyes for super-resolution imaging.J Biol Inorg Chem. 2017 Jul;22(5):639-652. doi: 10.1007/s00775-016-1435-y. Epub 2017 Jan 12. J Biol Inorg Chem. 2017. PMID: 28083655 Review.
-
Recent Advances in Fluorescence Imaging by Genetically Encoded Non-canonical Amino Acids.J Mol Biol. 2022 Apr 30;434(8):167248. doi: 10.1016/j.jmb.2021.167248. Epub 2021 Sep 20. J Mol Biol. 2022. PMID: 34547330 Review.
Cited by
-
Genetic Code Expansion: Recent Developments and Emerging Applications.Chem Rev. 2025 Jan 22;125(2):523-598. doi: 10.1021/acs.chemrev.4c00216. Epub 2024 Dec 31. Chem Rev. 2025. PMID: 39737807 Free PMC article. Review.
-
Biosynthesis of Halogenated Tryptophans for Protein Engineering Using Genetic Code Expansion.Chembiochem. 2024 Oct 16;25(20):e202400366. doi: 10.1002/cbic.202400366. Epub 2024 Sep 3. Chembiochem. 2024. PMID: 38958600 Free PMC article.
-
Genetic encoding of a highly photostable, long lifetime fluorescent amino acid for imaging in mammalian cells.Chem Sci. 2021 Aug 3;12(36):11955-11964. doi: 10.1039/d1sc01914g. eCollection 2021 Sep 22. Chem Sci. 2021. PMID: 34976337 Free PMC article.
-
A hydrophobic photouncaging reaction to profile the lipid droplet interactome in tissues.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2420861122. doi: 10.1073/pnas.2420861122. Epub 2025 Apr 16. Proc Natl Acad Sci U S A. 2025. PMID: 40238459
-
Noncanonical Amino Acid Tools and Their Application to Membrane Protein Studies.Chem Rev. 2024 Nov 27;124(22):12498-12550. doi: 10.1021/acs.chemrev.4c00181. Epub 2024 Nov 7. Chem Rev. 2024. PMID: 39509680 Free PMC article. Review.
References
-
- Puliti D, Warther D, Orange C, Specht A, Goeldner M, Bioorganic & Medicinal Chemistry 2011, 19, 1023–1029. - PubMed
-
- Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A, Angewandte Chemie International Edition 2012, 51, 8446–8476. - PubMed
-
- Chozinski TJ, Gagnon LA, Vaughan JC, FEBS Letters 2014, 588, 3603–3612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

