Site-Specific Incorporation of a Photoactivatable Fluorescent Amino Acid
- PMID: 32961013
- PMCID: PMC8011588
- DOI: 10.1002/cbic.202000602
Site-Specific Incorporation of a Photoactivatable Fluorescent Amino Acid
Abstract
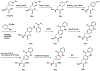
Photoactivatable fluorophores are emerging optical probes for biological applications. Most photoactivatable fluorophores are relatively large in size and need to be activated by ultraviolet light; this dramatically limits their applications. To introduce photoactivatable fluorophores into proteins, recent investigations have explored several protein-labeling technologies, including fluorescein arsenical hairpin (FlAsH) Tag, HaloTag labeling, SNAPTag labeling, and other bioorthogonal chemistry-based methods. However, these technologies require a multistep labeling process. Here, by using genetic code expansion and a single sulfur-for-oxygen atom replacement within an existing fluorescent amino acid, we have site-specifically incorporated the photoactivatable fluorescent amino acid thioacridonylalanine (SAcd) into proteins in a single step. Moreover, upon exposure to visible light, SAcd can be efficiently desulfurized to its oxo derivatives, thus restoring the strong fluorescence of labeled proteins.
Keywords: genetic code expansion; noncanonical amino acids; optical probes; photoactivatable fluorophores; protein labeling.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures


References
-
- Puliti D, Warther D, Orange C, Specht A, Goeldner M, Bioorganic & Medicinal Chemistry 2011, 19, 1023–1029. - PubMed
-
- Brieke C, Rohrbach F, Gottschalk A, Mayer G, Heckel A, Angewandte Chemie International Edition 2012, 51, 8446–8476. - PubMed
-
- Chozinski TJ, Gagnon LA, Vaughan JC, FEBS Letters 2014, 588, 3603–3612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

