Tracking Calcium Dynamics and Immune Surveillance at the Choroid Plexus Blood-Cerebrospinal Fluid Interface
- PMID: 32961128
- PMCID: PMC7847245
- DOI: 10.1016/j.neuron.2020.08.024
Tracking Calcium Dynamics and Immune Surveillance at the Choroid Plexus Blood-Cerebrospinal Fluid Interface
Abstract
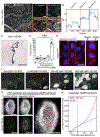
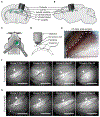
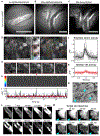
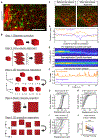
The choroid plexus (ChP) epithelium is a source of secreted signaling factors in cerebrospinal fluid (CSF) and a key barrier between blood and brain. Here, we develop imaging tools to interrogate these functions in adult lateral ventricle ChP in whole-mount explants and in awake mice. By imaging epithelial cells in intact ChP explants, we observed calcium activity and secretory events that increased in frequency following delivery of serotonergic agonists. Using chronic two-photon imaging in awake mice, we observed spontaneous subcellular calcium events as well as strong agonist-evoked calcium activation and cytoplasmic secretion into CSF. Three-dimensional imaging of motility and mobility of multiple types of ChP immune cells at baseline and following immune challenge or focal injury revealed a range of surveillance and defensive behaviors. Together, these tools should help illuminate the diverse functions of this understudied body-brain interface.
Keywords: calcium activity; cerebrospinal fluid; choroid plexus; epithelial cells; immune cells; secretion; serotonin; two-photon imaging.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
-
- Agnew WF, Yuen TG, and Achtyl TR (1980). Ultrastructural observations suggesting apocrine secretion in the choroid plexus: a comparative study. Neurol. Res 1, 313–332. - PubMed
-
- Ambudkar I (2018). Calcium signaling defects underlying salivary gland dysfunction. Biochim. Biophys. Acta Mol. Cell Res 1865 (11 Pt B), 1771–1777. - PubMed
-
- Audhya T, Adams JB, and Johansen L (2012). Correlation of serotonin levels in CSF, platelets, plasma, and urine. Biochim. Biophys. Acta 1820, 1496–1501. - PubMed
-
- Baddedy A, Rubak E, and Turner R (2015). Spatial Point Patterns: Methodology and Applications with R (Boca Raton, FL: Chapman and Hall/ CRC Press; ).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

