Persistent changes in extracellular lactate dynamics following synaptic potentiation
- PMID: 32961277
- PMCID: PMC7655607
- DOI: 10.1016/j.nlm.2020.107314
Persistent changes in extracellular lactate dynamics following synaptic potentiation
Abstract
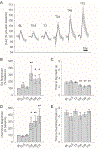
A diverse array of neurometabolic coupling mechanisms exist within the brain to ensure that sufficient metabolite availability is present to meet both acute and chronic energetic demands. Excitatory synaptic activity, which produces the majority of the brain's energetic demands, triggers a rapid metabolic response including a characteristic shift towards aerobic glycolysis. Herein, astrocytically derived lactate appears to serve as an important metabolite to meet the extensive metabolic needs of activated neurons. Despite a wealth of literature characterizing lactate's role in mediating these acute metabolic needs, the extent to which lactate supports chronic energetic demands of neurons remains unclear. We hypothesized that synaptic potentiation, a ubiquitous brain phenomenon that can produce chronic alterations in synaptic activity, could necessitate persistent alterations in brain energetics. In freely-behaving rats, we induced long-term potentiation (LTP) of synapses within the dentate gyrus through high-frequency electrical stimulation (HFS) of the medial perforant pathway. Before, during, and after LTP induction, we continuously recorded extracellular lactate concentrations within the dentate gyrus to assess how changes in synaptic strength alter local glycolytic activity. Synaptic potentiation 1) altered the acute response of extracellular lactate to transient neuronal activation as evident by a larger initial dip and subsequent overshoot and 2) chronically increased local lactate availability. Although synapses were potentiated immediately following HFS, observed changes in lactate dynamics were only evident beginning ~24 h later. Once observed, however, both synaptic potentiation and altered lactate dynamics persisted for the duration of the experiment (~72 h). Persistent alterations in synaptic strength, therefore, appear to be associated with metabolic plasticity in the form of persistent augmentation of glycolytic activity.
Keywords: Glycolysis; Hippocampus; Lactate; Long-term potentiation; Synaptic plasticity.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



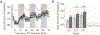
References
-
- Abraham WC, Williams JM (2008) LTP maintenance and its protein synthesis-dependence. Neurobiology of Learning and Memory 89:260–268. - PubMed
-
- Alle H, Roth A, Geiger JR (2009) Energy-efficient action potentials in hippocampal mossy fibers. Science 325:1405–1408. - PubMed
-
- Attwell D, Gibb A (2005) Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 6:841–849. - PubMed
-
- Aubert A, Costalat R (2005) Interaction between Astrocytes and Neurons Studied using a Mathematical Model of Compartmentalized Energy Metabolism: Journal of Cerebral Blood Flow & Metabolism 25:1476–1490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

