Oxidized Low-Density Lipoprotein Drives Dysfunction of the Liver Lymphatic System
- PMID: 32961356
- PMCID: PMC7803659
- DOI: 10.1016/j.jcmgh.2020.09.007
Oxidized Low-Density Lipoprotein Drives Dysfunction of the Liver Lymphatic System
Abstract
Background and aims: As the incidence of nonalcoholic steatohepatitis (NASH) continues to rise, understanding how normal liver functions are affected during disease is required before developing novel therapeutics which could reduce morbidity and mortality. However, very little is understood about how the transport of proteins and cells from the liver by the lymphatic vasculature is affected by inflammatory mediators or during disease.
Methods: To answer these questions, we utilized a well-validated mouse model of NASH and exposure to highly oxidized low density lipoprotein (oxLDL). In addition to single cell sequencing, multiplexed immunofluorescence and metabolomic analysis of liver lymphatic endothelial cells (LEC)s we evaluated lymphatic permeability and transport both in vitro and in vivo.
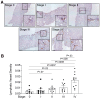
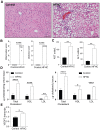
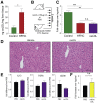
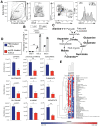
Results: Confirming similarities between human and mouse liver lymphatic vasculature in NASH, we found that the lymphatic vasculature expands as disease progresses and results in the downregulation of genes important to lymphatic identity and function. We also demonstrate, in mice with NASH, that fluorescein isothiocyanate (FITC) dextran does not accumulate in the liver draining lymph node upon intrahepatic injection, a defect that was rescued with therapeutic administration of the lymphatic growth factor, recombinant vascular endothelial growth factor C (rVEGFC). Similarly, exposure to oxLDL reduced the amount of FITC-dextran in the portal draining lymph node and through an LEC monolayer. We provide evidence that the mechanism by which oxLDL impacts lymphatic permeability is via a reduction in Prox1 expression which decreases lymphatic specific gene expression, impedes LEC metabolism and reorganizes the highly permeable lymphatic cell-cell junctions which are a defining feature of lymphatic capillaries.
Conclusions: We identify oxLDL as a major contributor to decreased lymphatic permeability in the liver, a change which is consistent with decreased protein homeostasis and increased inflammation during chronic liver disease.
Keywords: Inflammation; Lymphangiogenesis; Oxidized LDL; Permeability; VEGFC.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







Comment in
-
Lymphatic Dysfunction as a Novel Therapeutic Target in Nonalcoholic Steatohepatitis.Cell Mol Gastroenterol Hepatol. 2021;11(2):663-664. doi: 10.1016/j.jcmgh.2020.10.013. Epub 2020 Nov 18. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33220266 Free PMC article. No abstract available.
References
-
- Noureddin M., Vipani A., Bresee C., Todo T., Kim I.K., Alkhouri N., Setiawan V.W., Tran T., Ayoub W.S., Lu S.C., Klein A.S., Sundaram V., Nissen N.N. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. - PMC - PubMed
-
- Lim H.Y., Thiam C.H., Yeo K.P., Bisoendial R., Hii C.S., McGrath K.C., Tan K.W., Heather A., Alexander J.S., Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

