Mitochondrial dynamics: Shaping and remodeling an organelle network
- PMID: 32961383
- PMCID: PMC7925334
- DOI: 10.1016/j.ceb.2020.08.014
Mitochondrial dynamics: Shaping and remodeling an organelle network
Abstract
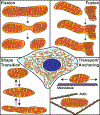
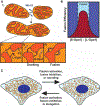
Mitochondria form networks that continually remodel and adapt to carry out their cellular function. The mitochondrial network is remodeled through changes in mitochondrial morphology, number, and distribution within the cell. Mitochondrial dynamics depend directly on fission, fusion, shape transition, and transport or tethering along the cytoskeleton. Over the past several years, many of the mechanisms underlying these processes have been uncovered. It has become clear that each process is precisely and contextually regulated within the cell. Here, we discuss the mechanisms regulating each aspect of mitochondrial dynamics, which together shape the network as a whole.
Keywords: Cytoskeleton; Fission; Fusion; Mitochondria; Morphology; Transport.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement Nothing declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

