Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles
- PMID: 32961738
- PMCID: PMC7560102
- DOI: 10.3390/pharmaceutics12090892
Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles
Abstract
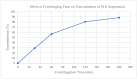
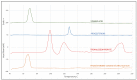
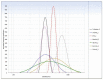
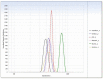
Cryoprotectants are often required in lyophilization to reduce or eliminate agglomeration of solute or suspended materials. The aim of this study was to select a cryoprotecting agent and optimize its concentration in a solid lipid nanoparticle formulation. Progesterone-loaded stearic acid solid lipid nanoparticles (SA-P SLNs) were prepared by hot homogenization with high speed mixing and sonication. The stearic acid content was 4.6% w/w and progesterone was 0.46% w/w of the initial formulation. Multiple surfactants were evaluated, and a lecithin and sodium taurocholate system was chosen. Three concentrations of surfactant were then evaluated, and a concentration of 2% w/w was chosen based on particle size, polydispersity, and zeta potential. Agglomeration of SA-P SLNs after lyophilization was observed as measured by increased particle size. Dextran, glycine, mannitol, polyvinylpyrrolidone (PVP), sorbitol, and trehalose were evaluated as cryoprotectants by both an initial freeze-thaw analysis and after lyophilization. Once selected as the cryoprotectant, trehalose was evaluated at 5%, 10%, 15%, and 20% for optimal concentration, with 20% trehalose being finally selected as the level of choice. Evaluation by DSC confirmed intimate interaction between stearic acid and progesterone in the SA-P SLNs, and polarized light microscopy shows successful lyophilization of the trehalose/SA-P SLN. A short term 28-day stability study suggests the need for refrigeration of the final lyophilized SA-P SLNs in moisture vapor impermeable packaging.
Keywords: cryoprotectant; freezing rate; lyophilization; solid lipid nanoparticles; surfactants; trehalose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Development of highly stable nifedipine solid-lipid nanoparticles.Chem Pharm Bull (Tokyo). 2014;62(5):399-406. doi: 10.1248/cpb.c13-00684. Chem Pharm Bull (Tokyo). 2014. PMID: 24789922
-
Development of Houttuynia cordata Extract-Loaded Solid Lipid Nanoparticles for Oral Delivery: High Drug Loading Efficiency and Controlled Release.Molecules. 2017 Dec 13;22(12):2215. doi: 10.3390/molecules22122215. Molecules. 2017. PMID: 29236057 Free PMC article.
-
Annealing as a tool for the optimization of lyophilization and ensuring of the stability of protein-loaded PLGA nanoparticles.Int J Pharm. 2016 Apr 30;503(1-2):163-73. doi: 10.1016/j.ijpharm.2016.03.011. Epub 2016 Mar 10. Int J Pharm. 2016. PMID: 26972381
-
Optimization of the lyophilization process for long-term stability of solid-lipid nanoparticles.Drug Dev Ind Pharm. 2012 Oct;38(10):1270-9. doi: 10.3109/03639045.2011.645835. Epub 2012 Jan 12. Drug Dev Ind Pharm. 2012. PMID: 22235767
-
Armamentarium of Cryoprotectants in Peptide Vaccines: Mechanistic Insight, Challenges, Opportunities and Future Prospects.Int J Pept Res Ther. 2021;27(4):2965-2982. doi: 10.1007/s10989-021-10303-y. Epub 2021 Oct 19. Int J Pept Res Ther. 2021. PMID: 34690621 Free PMC article. Review.
Cited by
-
Manufacturing Bacteriophages (Part 2 of 2): Formulation, Analytics and Quality Control Considerations.Pharmaceuticals (Basel). 2021 Sep 2;14(9):895. doi: 10.3390/ph14090895. Pharmaceuticals (Basel). 2021. PMID: 34577595 Free PMC article. Review.
-
Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms.Pharmaceuticals (Basel). 2023 Mar 22;16(3):474. doi: 10.3390/ph16030474. Pharmaceuticals (Basel). 2023. PMID: 36986574 Free PMC article. Review.
-
Development of a Lyophilized Off-the-Shelf Mesenchymal Stem Cell-Derived Acellular Therapeutic.Pharmaceutics. 2022 Apr 13;14(4):849. doi: 10.3390/pharmaceutics14040849. Pharmaceutics. 2022. PMID: 35456683 Free PMC article.
-
Different Size Formulations of Fluopyram: Preparation, Antifungal Activity, and Accumulation in the Fungal Pathogen Botrytis cinerea.Molecules. 2023 Aug 17;28(16):6099. doi: 10.3390/molecules28166099. Molecules. 2023. PMID: 37630351 Free PMC article.
-
Eplerenone nanocrystals engineered by controlled crystallization for enhanced oral bioavailability.Drug Deliv. 2021 Dec;28(1):2510-2524. doi: 10.1080/10717544.2021.2008051. Drug Deliv. 2021. PMID: 34842018 Free PMC article.
References
-
- Danaei M., Dehghankhold M., Ataei S., Davarani F.H., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

