Non-Viral Targeted Nucleic Acid Delivery: Apply Sequences for Optimization
- PMID: 32961908
- PMCID: PMC7559072
- DOI: 10.3390/pharmaceutics12090888
Non-Viral Targeted Nucleic Acid Delivery: Apply Sequences for Optimization
Abstract
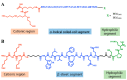
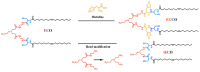
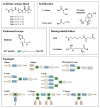
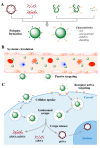
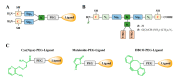
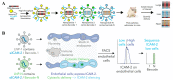
In nature, genomes have been optimized by the evolution of their nucleic acid sequences. The design of peptide-like carriers as synthetic sequences provides a strategy for optimizing multifunctional targeted nucleic acid delivery in an iterative process. The optimization of sequence-defined nanocarriers differs for different nucleic acid cargos as well as their specific applications. Supramolecular self-assembly enriched the development of a virus-inspired non-viral nucleic acid delivery system. Incorporation of DNA barcodes presents a complementary approach of applying sequences for nanocarrier optimization. This strategy may greatly help to identify nucleic acid carriers that can overcome pharmacological barriers and facilitate targeted delivery in vivo. Barcode sequences enable simultaneous evaluation of multiple nucleic acid nanocarriers in a single test organism for in vivo biodistribution as well as in vivo bioactivity.
Keywords: DNA-barcode; non-viral; nucleic acid delivery; peptide; pharmacological barriers; sequence-defined; supramolecular self-assembly; tumor-targeted.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Tabernero J., Shapiro G.I., LoRusso P.M., Cervantes A., Schwartz G.K., Weiss G.J., Paz-Ares L., Cho D.C., Infante J.R., Alsina M. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

